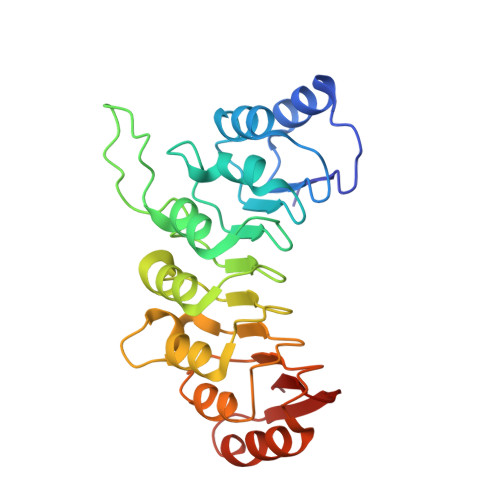
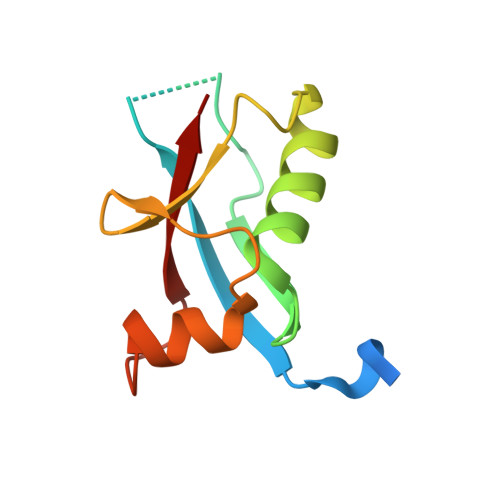
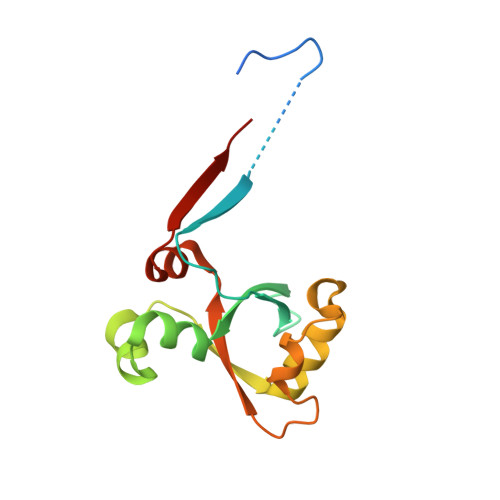
Calcium modulates the tethering of BCOR-PRC1.1 enzymatic core to KDM2B via liquid-liquid phase separation.
Chen, R., Shen, F., Zhang, Y., Sun, M., Dong, Y., Yin, Y., Su, C., Peng, C., Liu, J., Xu, J.(2024) Commun Biol 7: 1112-1112
- PubMed: 39256555
- DOI: https://doi.org/10.1038/s42003-024-06820-3
- Primary Citation of Related Structures:
8HCU - PubMed Abstract:
Recruitment of non-canonical BCOR-PRC1.1 to non-methylated CpG islands via KDM2B plays a fundamental role in transcription control during developmental processes and cancer progression. However, the mechanism is still largely unknown on how this recruitment is regulated. Here, we unveiled the importance of the Poly-D/E regions within the linker of BCOR for its binding to KDM2B. Interestingly, we also demonstrated that these negatively charged Poly-D/E regions on BCOR play autoinhibitory roles in liquid-liquid phase separation (LLPS) of BCOR ANK-linker-PUFD /PCGF1 RAWUL . Through neutralizing negative charges of these Poly-D/E regions, Ca 2+ not only weakens the interaction between BCOR/PCGF1 and KDM2B, but also promotes co-condensation of the enzymatic core of BCOR-PRC1.1 with KDM2B into liquid-like droplet. Accordingly, we propose that Ca 2+ could modulate the compartmentation and recruitment of the enzymatic core of BCOR-PRC1.1 on KDM2B target loci. Thus, our finding advances the mechanistic understanding on how the tethering of BCOR-PRC1.1 enzymatic core to KDM2B is regulated.
- Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, 230026, China.
Organizational Affiliation: