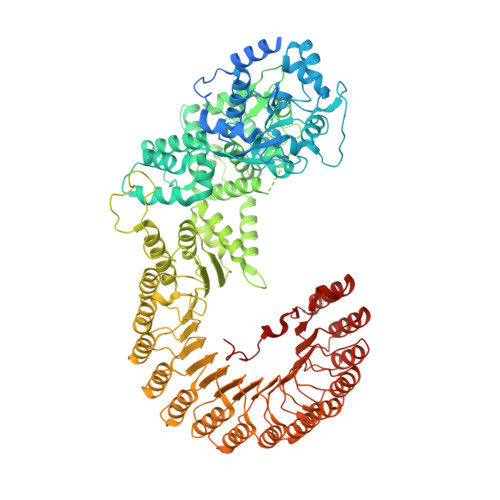
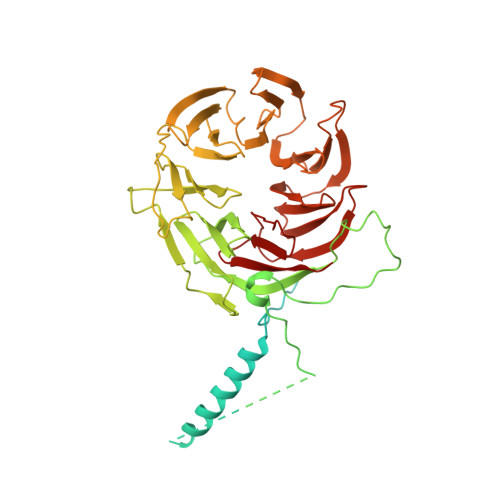
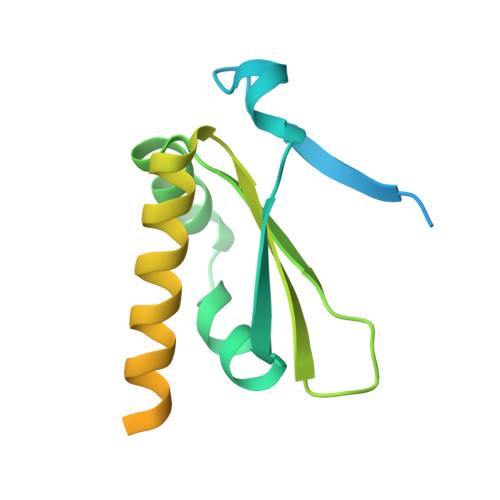
Structural basis of the subcortical maternal complex and its implications in reproductive disorders.
Chi, P., Ou, G., Qin, D., Han, Z., Li, J., Xiao, Q., Gao, Z., Xu, C., Qi, Q., Liu, Q., Liu, S., Li, J., Guo, L., Lu, Y., Chen, J., Wang, X., Shi, H., Li, L., Deng, D.(2024) Nat Struct Mol Biol 31: 115-124
- PubMed: 38177687
- DOI: https://doi.org/10.1038/s41594-023-01153-x
- Primary Citation of Related Structures:
8H93, 8H94, 8H95, 8H96 - PubMed Abstract:
The subcortical maternal complex (SCMC) plays a crucial role in early embryonic development. Malfunction of SCMC leads to reproductive diseases in women. However, the molecular function and assembly basis for SCMC remain elusive. Here we reconstituted mouse SCMC and solved the structure at atomic resolution using single-particle cryo-electron microscopy. The core complex of SCMC was formed by MATER, TLE6 and FLOPED, and MATER embraced TLE6 and FLOPED via its NACHT and LRR domains. Two core complexes further dimerize through interactions between two LRR domains of MATERs in vitro. FILIA integrates into SCMC by interacting with the carboxyl-terminal region of FLOPED. Zygotes from mice with Floped C-terminus truncation showed delayed development and resembled the phenotype of zygotes from Filia knockout mice. More importantly, the assembly of mouse SCMC was affected by corresponding clinical variants associated with female reproductive diseases and corresponded with a prediction based on the mouse SCMC structure. Our study paves the way for further investigations on SCMC functions during mammalian preimplantation embryonic development and reveals underlying causes of female reproductive diseases related to SCMC mutations, providing a new strategy for the diagnosis of female reproductive disorders.
- Department of Obstetrics, Key Laboratory of Birth Defects and Related Disease of Women and Children of MOE, State Key Laboratory of Biotherapy, West China Second Hospital, Sichuan University, Chengdu, China.
Organizational Affiliation: