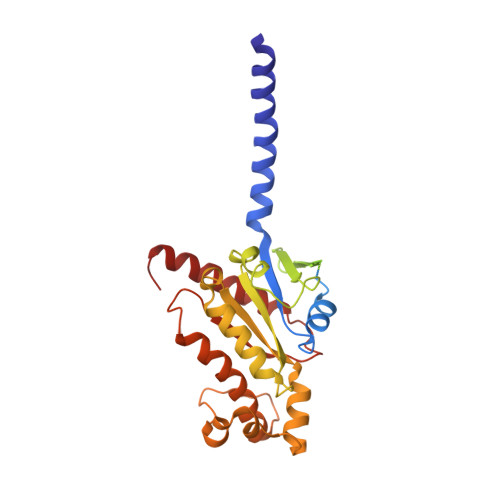
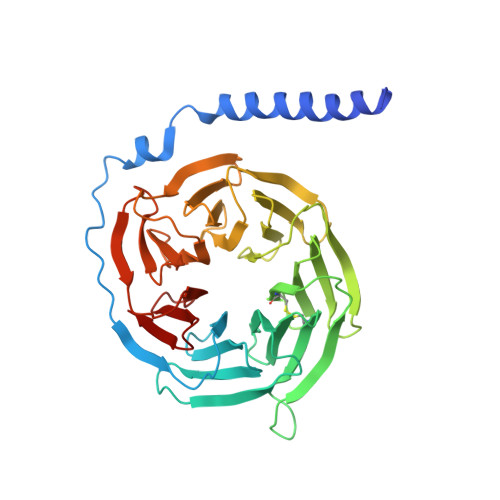
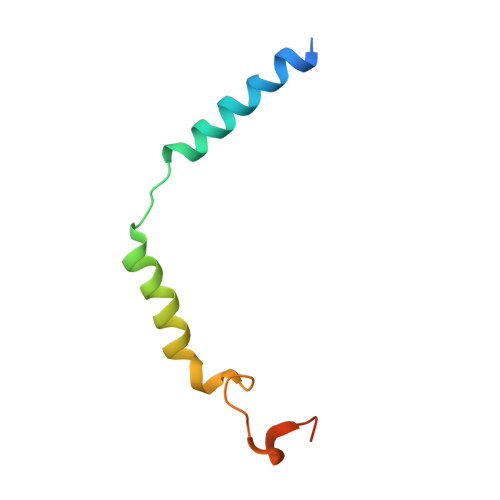
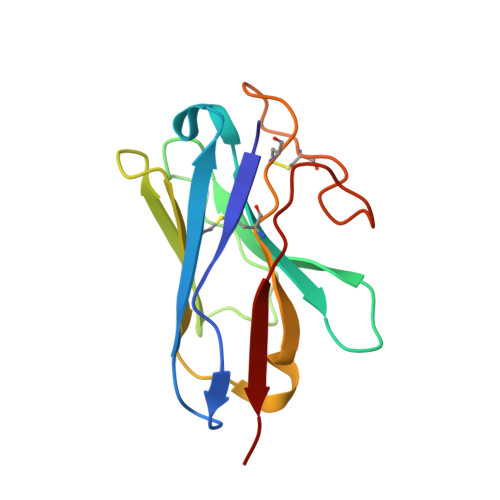
Class B1 GPCR activation by an intracellular agonist.
Kobayashi, K., Kawakami, K., Kusakizako, T., Tomita, A., Nishimura, M., Sawada, K., Okamoto, H.H., Hiratsuka, S., Nakamura, G., Kuwabara, R., Noda, H., Muramatsu, H., Shimizu, M., Taguchi, T., Inoue, A., Murata, T., Nureki, O.(2023) Nature 618: 1085-1093
- PubMed: 37286611
- DOI: https://doi.org/10.1038/s41586-023-06169-3
- Primary Citation of Related Structures:
8GW8 - PubMed Abstract:
G protein-coupled receptors (GPCRs) generally accommodate specific ligands in the orthosteric-binding pockets. Ligand binding triggers a receptor allosteric conformational change that leads to the activation of intracellular transducers, G proteins and β-arrestins. Because these signals often induce adverse effects, the selective activation mechanism for each transducer must be elucidated. Thus, many orthosteric-biased agonists have been developed, and intracellular-biased agonists have recently attracted broad interest. These agonists bind within the receptor intracellular cavity and preferentially tune the specific signalling pathway over other signalling pathways, without allosteric rearrangement of the receptor from the extracellular side 1-3 . However, only antagonist-bound structures are currently available 1,4-6 , and there is no evidence to support that biased agonist binding occurs within the intracellular cavity. This limits the comprehension of intracellular-biased agonism and potential drug development. Here we report the cryogenic electron microscopy structure of a complex of G s and the human parathyroid hormone type 1 receptor (PTH1R) bound to a PTH1R agonist, PCO371. PCO371 binds within an intracellular pocket of PTH1R and directly interacts with G s . The PCO371-binding mode rearranges the intracellular region towards the active conformation without extracellularly induced allosteric signal propagation. PCO371 stabilizes the significantly outward-bent conformation of transmembrane helix 6, which facilitates binding to G proteins rather than β-arrestins. Furthermore, PCO371 binds within the highly conserved intracellular pocket, activating 7 out of the 15 class B1 GPCRs. Our study identifies a new and conserved intracellular agonist-binding pocket and provides evidence of a biased signalling mechanism that targets the receptor-transducer interface.
- Department of Biological Sciences, Graduate School of Science, The University of Tokyo, Tokyo, Japan.
Organizational Affiliation: