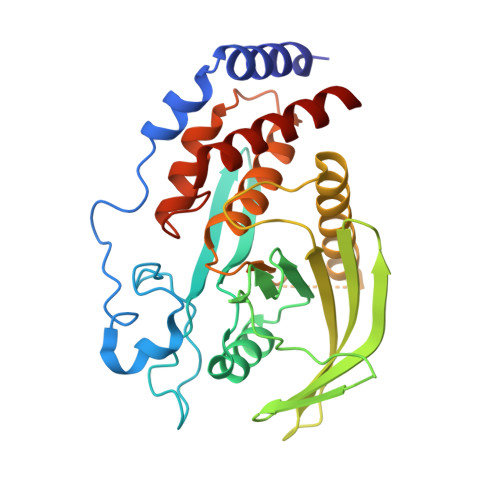
Structural analysis of PTPN21 reveals a dominant-negative effect of the FERM domain on its phosphatase activity.
Chen, L., Qian, Z., Zheng, Y., Zhang, J., Sun, J., Zhou, C., Xiao, H.(2024) Sci Adv 10: eadi7404-eadi7404
- PubMed: 38416831
- DOI: https://doi.org/10.1126/sciadv.adi7404
- Primary Citation of Related Structures:
8GVL, 8GVV, 8GWH, 8GXE - PubMed Abstract:
PTPN21 belongs to the four-point-one, ezrin, radixin, moesin (FERM) domain-containing protein tyrosine phosphatases (PTP) and plays important roles in cytoskeleton-associated cellular processes like cell adhesion, motility, and cargo transport. Because of the presence of a WPE loop instead of a WPD loop in the phosphatase domain, it is often considered to lack phosphatase activity. However, many of PTPN21's biological functions require its catalytic activity. To reconcile these findings, we have determined the structures of individual PTPN21 FERM, PTP domains, and a complex between FERM-PTP. Combined with biochemical analysis, we have found that PTPN21 PTP is weakly active and is autoinhibited by association with its FERM domain. Disruption of FERM-PTP interaction results in enhanced ERK activation. The oncogenic HPV18 E7 protein binds to PTP at the same location as PTPN21 FERM, indicating that it may act by displacing the FERM domain from PTP. Our results provide mechanistic insight into PTPN21 and benefit functional studies of PTPN21-mediated processes.
- Department of Pathology of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310016, China.
Organizational Affiliation: