Noncanonical interactions and conformational dynamics in cargo-Pex5-Pex14 ternary complex for peroxisomal import
Sonani, R.R., Blat, A., Jemiola-Rzeminska, M., Lipinski, O., Patel, S.N., Sood, T., Dubin, G.(2023) bioRxiv
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2023) bioRxiv
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Peroxisomal membrane protein PEX14 | A [auth F] | 372 | Trypanosoma cruzi | Mutation(s): 0 Gene Names: C3747_80g13 |  |
UniProt | |||||
Find proteins for A0A2V2WKZ5 (Trypanosoma cruzi) Explore A0A2V2WKZ5 Go to UniProtKB: A0A2V2WKZ5 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A2V2WKZ5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| malate dehydrogenase | B [auth A], C [auth B], D [auth C], E [auth D] | 323 | Trypanosoma cruzi strain CL Brener | Mutation(s): 0 Gene Names: Tc00.1047053506503.69 EC: 1.1.1.37 | 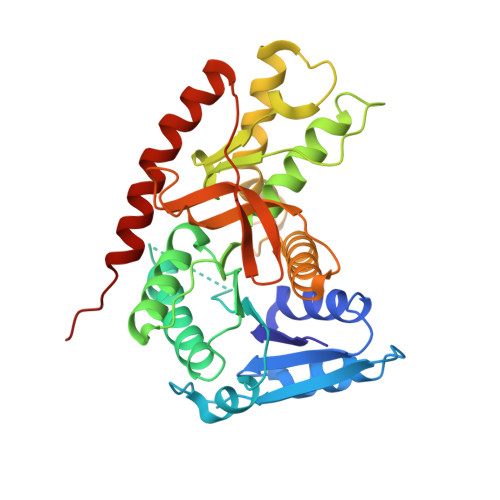 |
UniProt | |||||
Find proteins for Q4DRD8 (Trypanosoma cruzi (strain CL Brener)) Explore Q4DRD8 Go to UniProtKB: Q4DRD8 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q4DRD8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Peroxisome targeting signal 1 receptor | F [auth E] | 666 | Trypanosoma cruzi cruzi | Mutation(s): 0 Gene Names: TCDM_08436 | 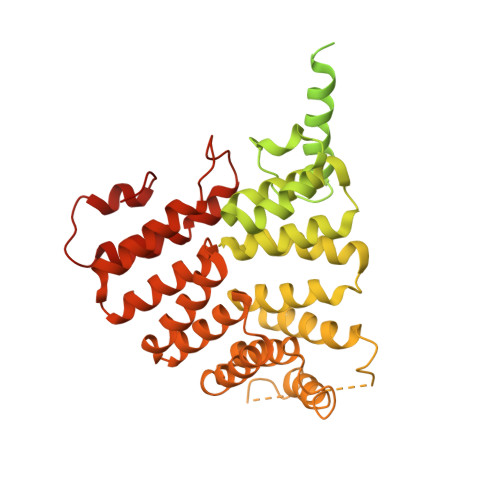 |
UniProt | |||||
Find proteins for V5B7T1 (Trypanosoma cruzi Dm28c) Explore V5B7T1 Go to UniProtKB: V5B7T1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | V5B7T1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.20.1_4487: |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Polish National Science Centre | Poland | 2020/39/B/NZ1/01551 |
| Foundation for Polish Science | Poland | TEAM TECH CORE FACILITY/2017-4/6 |