Isosteric improvements to liver receptor homolog-1 small molecule modulators
Cato, M.L., Abraham, S.M., Spurlin, R.M., Flynn, A.R., Colucci, J.K., D'Agostino, E.H., Johnson, A.M., Jui, N.T., Ortlund, E.A.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Nuclear receptor subfamily 5 group A member 2 | 246 | Homo sapiens | Mutation(s): 0 Gene Names: NR5A2, B1F, CPF, FTF | 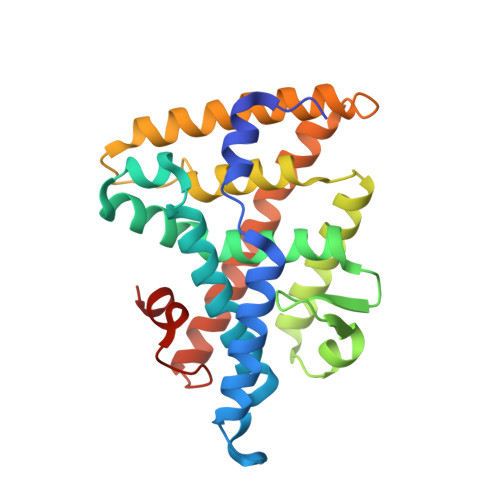 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O00482 (Homo sapiens) Explore O00482 Go to UniProtKB: O00482 | |||||
PHAROS: O00482 GTEx: ENSG00000116833 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O00482 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Nuclear receptor coactivator 2 | 14 | Homo sapiens | Mutation(s): 0 |  | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q15596 (Homo sapiens) Explore Q15596 Go to UniProtKB: Q15596 | |||||
PHAROS: Q15596 GTEx: ENSG00000140396 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q15596 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| XKE (Subject of Investigation/LOI) Query on XKE | C [auth A] | (1~{R},3~{a}~{R},6~{a}~{R})-4-phenyl-3~{a}-(1-phenylethenyl)-5-[9-(1~{H}-1,2,3,4-tetrazol-5-yl)nonyl]-2,3,6,6~{a}-tetrahydro-1~{H}-pentalen-1-ol C32 H40 N4 O KWRMCWKEHBRILN-LBRLCBGXSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 89.161 | α = 90 |
| b = 89.161 | β = 90 |
| c = 105.692 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Disease (NIH/NIDDK) | United States | F31-DK122745 |
| National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Disease (NIH/NIDDK) | United States | R01-DK115213 |
| American Heart Association | United States | 20PRE35200311 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | T32-GM008367-29 |
| National Science Foundation (NSF, United States) | United States | DGE-1444932 |