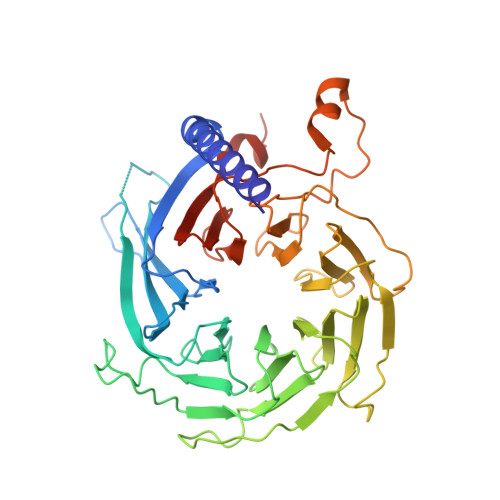
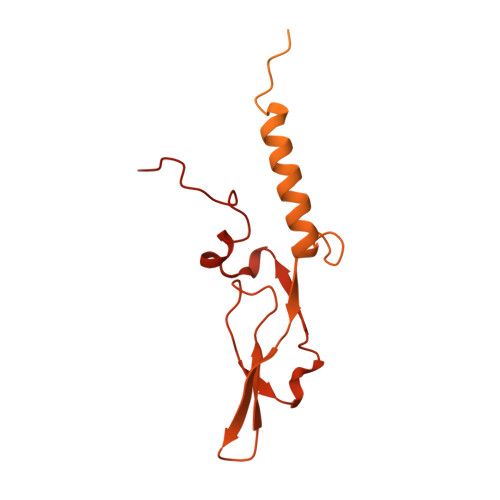
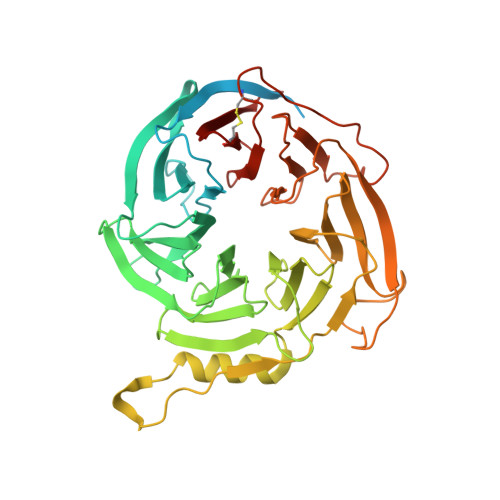
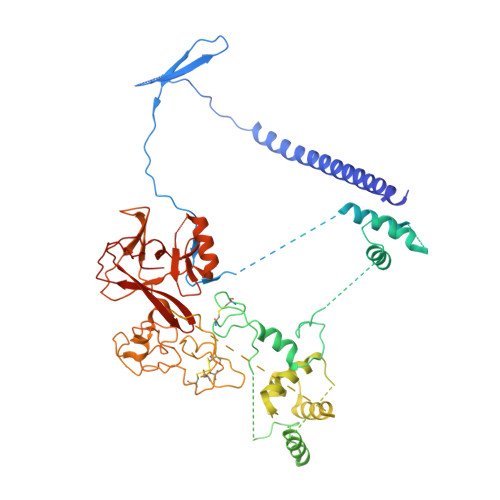
Auto-inhibition of PRC2 by the broadly expressed long isoform of AEBP2.
Mucha, M., Lai, Z., McKenzie, N.J., Matra, F., Boudes, M., Flanigan, S.F., Alejo-Vinogradova, M.T., Monger, C., Zhang, Q., Nimmo, D., Healy, E., Silva, A.J., Angelov, D., Reck, D.M., Holland, G., Atmaca, Z.E., King, H.E., Hamilton, M., Glancy, E., Nolan, J., Weatheritt, R.J., Bell, O., Vermeulen, M., Davidovich, C., Bracken, A.P.(2025) EMBO J
- PubMed: 41168462
- DOI: https://doi.org/10.1038/s44318-025-00616-9
- Primary Citation of Related Structures:
8EQV - PubMed Abstract:
Polycomb Repressive Complex 2 (PRC2) is an essential chromatin regulator responsible for mono-, di- and tri-methylating H3K27. Control of PRC2 activity is a critical process in development and disease, yet no inhibitory cofactor has been identified in somatic cells. Here, we show that the alternative isoforms of its accessory subunit AEBP2, namely AEBP2 S (short) and AEBP2 L (long), perform opposite functions in modulating PRC2 activity. Contrary to prior assumptions that AEBP2 enhances PRC2 function, we find that the widely expressed AEBP2 L isoform inhibits it. AEBP2 L is expressed throughout embryogenesis and adulthood and inhibits PRC2 DNA binding, histone methyltransferase activity, and binding to target genes. In contrast, AEBP2 S , expressed during early embryogenesis, promotes PRC2 DNA-binding activity and is essential for de novo repression of target genes during the transition from naïve to primed pluripotency. Mechanistically, through high-resolution cryo-EM and mutagenesis, we show that the recently evolved, negatively charged N-terminal region of AEBP2 L inhibits PRC2. We propose a scenario in which the N-terminus of AEBP2 L arose in vertebrates to restrain PRC2 activity in somatic cells.
- Smurfit Institute of Genetics, Trinity College Dublin, Dublin, Ireland.
Organizational Affiliation: