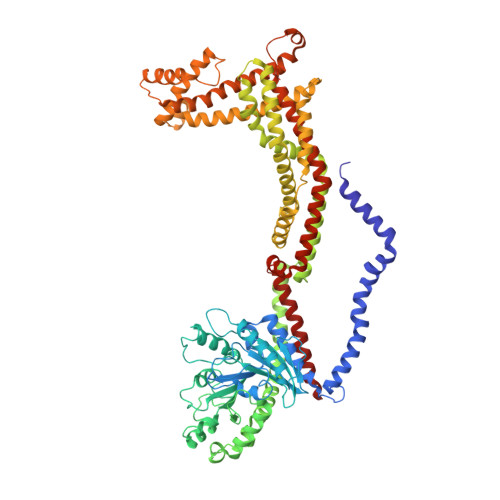
OPA1 helical structures give perspective to mitochondrial dysfunction.
Nyenhuis, S.B., Wu, X., Strub, M.P., Yim, Y.I., Stanton, A.E., Baena, V., Syed, Z.A., Canagarajah, B., Hammer, J.A., Hinshaw, J.E.(2023) Nature 620: 1109-1116
- PubMed: 37612506
- DOI: https://doi.org/10.1038/s41586-023-06462-1
- Primary Citation of Related Structures:
8EEW, 8EF7, 8EFF, 8EFR, 8EFS, 8EFT - PubMed Abstract:
Dominant optic atrophy is one of the leading causes of childhood blindness. Around 60-80% of cases 1 are caused by mutations of the gene that encodes optic atrophy protein 1 (OPA1), a protein that has a key role in inner mitochondrial membrane fusion and remodelling of cristae and is crucial for the dynamic organization and regulation of mitochondria 2 . Mutations in OPA1 result in the dysregulation of the GTPase-mediated fusion process of the mitochondrial inner and outer membranes 3 . Here we used cryo-electron microscopy methods to solve helical structures of OPA1 assembled on lipid membrane tubes, in the presence and absence of nucleotide. These helical assemblies organize into densely packed protein rungs with minimal inter-rung connectivity, and exhibit nucleotide-dependent dimerization of the GTPase domains-a hallmark of the dynamin superfamily of proteins 4 . OPA1 also contains several unique secondary structures in the paddle domain that strengthen its membrane association, including membrane-inserting helices. The structural features identified in this study shed light on the effects of pathogenic point mutations on protein folding, inter-protein assembly and membrane interactions. Furthermore, mutations that disrupt the assembly interfaces and membrane binding of OPA1 cause mitochondrial fragmentation in cell-based assays, providing evidence of the biological relevance of these interactions.
- Laboratory of Cell and Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, NIH, Bethesda, MD, USA.
Organizational Affiliation: