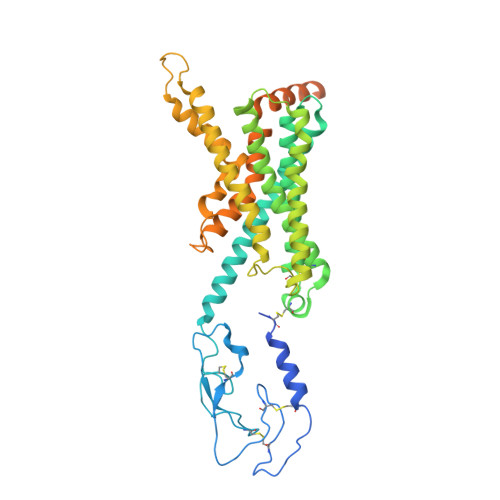
Understanding VPAC receptor family peptide binding and selectivity.
Piper, S.J., Deganutti, G., Lu, J., Zhao, P., Liang, Y.L., Lu, Y., Fletcher, M.M., Hossain, M.A., Christopoulos, A., Reynolds, C.A., Danev, R., Sexton, P.M., Wootten, D.(2022) Nat Commun 13: 7013-7013
- PubMed: 36385145
- DOI: https://doi.org/10.1038/s41467-022-34629-3
- Primary Citation of Related Structures:
8E3X, 8E3Y, 8E3Z - PubMed Abstract:
The vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) receptors are key regulators of neurological processes. Despite recent structural data, a comprehensive understanding of peptide binding and selectivity among different subfamily receptors is lacking. Here, we determine structures of active, Gs-coupled, VIP-VPAC1R, PACAP27-VPAC1R, and PACAP27-PAC1R complexes. Cryo-EM structural analyses and molecular dynamics simulations (MDSs) reveal fewer stable interactions between VPAC1R and VIP than for PACAP27, more extensive dynamics of VIP interaction with extracellular loop 3, and receptor-dependent differences in interactions of conserved N-terminal peptide residues with the receptor core. MD of VIP modelled into PAC1R predicts more transient VIP-PAC1R interactions in the receptor core, compared to VIP-VPAC1R, which may underlie the selectivity of VIP for VPAC1R over PAC1R. Collectively, our work improves molecular understanding of peptide engagement with the PAC1R and VPAC1R that may benefit the development of novel selective agonists.
- Drug Discovery Biology, Monash Institute of Pharmaceutical Sciences, Monash University, Parkville, 3052, VIC, Australia.
Organizational Affiliation: