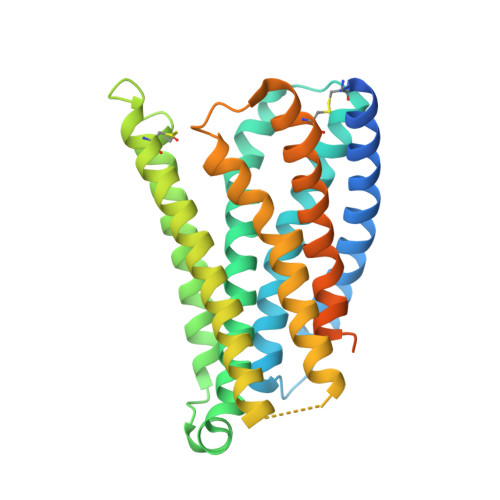
Ligand recognition and allosteric modulation of the human MRGPRX1 receptor.
Liu, Y., Cao, C., Huang, X.P., Gumpper, R.H., Rachman, M.M., Shih, S.L., Krumm, B.E., Zhang, S., Shoichet, B.K., Fay, J.F., Roth, B.L.(2023) Nat Chem Biol 19: 416-422
- PubMed: 36302898
- DOI: https://doi.org/10.1038/s41589-022-01173-6
- Primary Citation of Related Structures:
8DWC, 8DWG, 8DWH - PubMed Abstract:
The human MAS-related G protein-coupled receptor X1 (MRGPRX1) is preferentially expressed in the small-diameter primary sensory neurons and involved in the mediation of nociception and pruritus. Central activation of MRGPRX1 by the endogenous opioid peptide fragment BAM8-22 and its positive allosteric modulator ML382 has been shown to effectively inhibit persistent pain, making MRGPRX1 a promising target for non-opioid pain treatment. However, the activation mechanism of MRGPRX1 is still largely unknown. Here we report three high-resolution cryogenic electron microscopy structures of MRGPRX1-Gαq in complex with BAM8-22 alone, with BAM8-22 and ML382 simultaneously as well as with a synthetic agonist compound-16. These structures reveal the agonist binding mode for MRGPRX1 and illuminate the structural requirements for positive allosteric modulation. Collectively, our findings provide a molecular understanding of the activation and allosteric modulation of the MRGPRX1 receptor, which could facilitate the structure-based design of non-opioid pain-relieving drugs.
- Department of Pharmacology, University of North Carolina School of Medicine, Chapel Hill, NC, USA.
Organizational Affiliation: