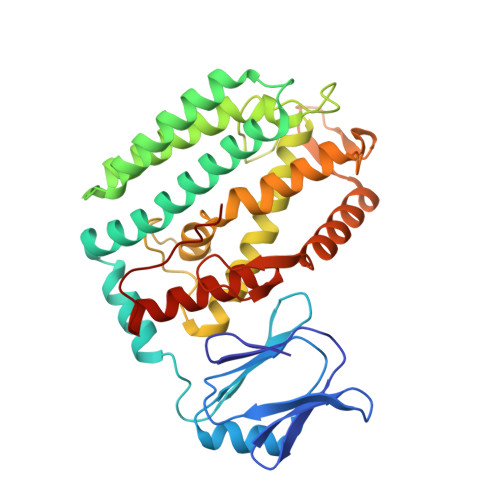
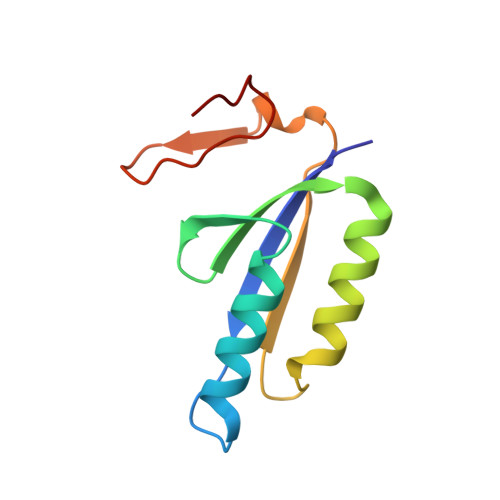
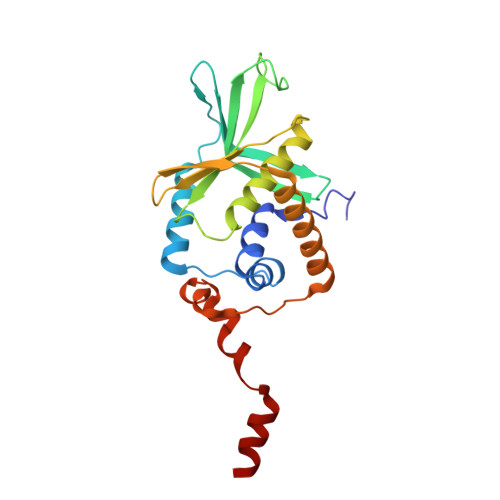
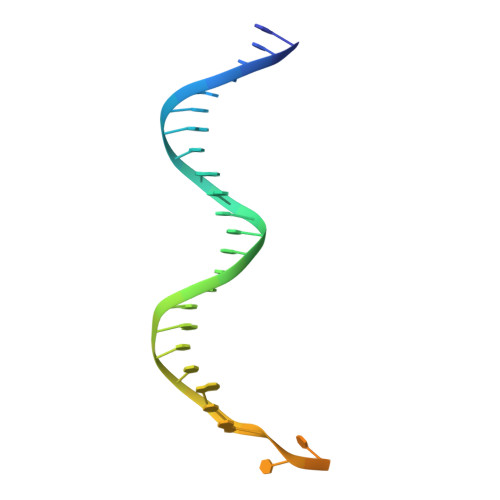
PAM binding ensures orientational integration during Cas4-Cas1-Cas2-mediated CRISPR adaptation.
Dhingra, Y., Suresh, S.K., Juneja, P., Sashital, D.G.(2022) Mol Cell 82: 4353
- PubMed: 36272411
- DOI: https://doi.org/10.1016/j.molcel.2022.09.030
- Primary Citation of Related Structures:
8D3L, 8D3M, 8D3P, 8D3Q - PubMed Abstract:
Adaptation in CRISPR-Cas systems immunizes bacteria and archaea against mobile genetic elements. In many DNA-targeting systems, the Cas4-Cas1-Cas2 complex is required for selection and processing of DNA segments containing PAM sequences prior to integration of these "prespacer" substrates as spacers in the CRISPR array. We determined cryo-EM structures of the Cas4-Cas1-Cas2 adaptation complex from the type I-C system that encodes standalone Cas1 and Cas4 proteins. The structures reveal how Cas4 specifically reads out bases within the PAM sequence and how interactions with both Cas1 and Cas2 activate Cas4 endonuclease activity. The Cas4-PAM interaction ensures tight binding between the adaptation complex and the prespacer, significantly enhancing integration of the non-PAM end into the CRISPR array and ensuring correct spacer orientation. Corroborated with our biochemical results, Cas4-Cas1-Cas2 structures with substrates representing various stages of CRISPR adaptation reveal a temporally resolved mechanism for maturation and integration of functional spacers into the CRISPR array.
- Roy J. Carver Department of Biochemistry, Biophysics, & Molecular Biology, Iowa State University, Ames, IA, USA.
Organizational Affiliation: