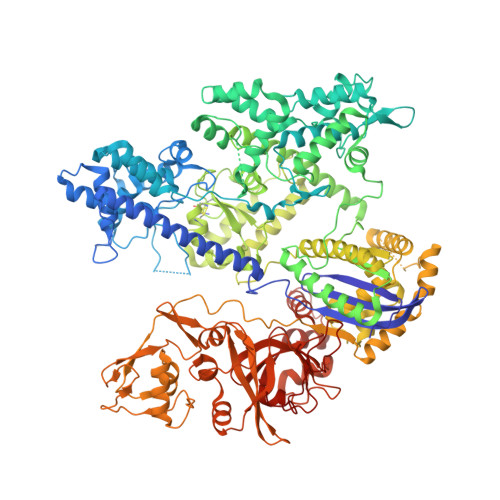
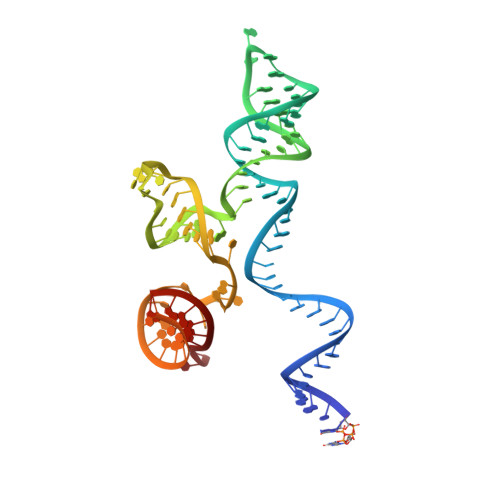
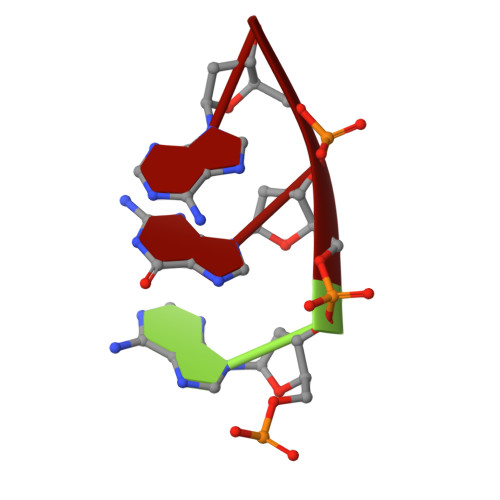
Coupled catalytic states and the role of metal coordination in Cas9.
Das, A., Rai, J., Roth, M.O., Shu, Y., Medina, M.L., Barakat, M.R., Li, H.(2023) Nat Catal 6: 969-977
- PubMed: 38348449
- DOI: https://doi.org/10.1038/s41929-023-01031-1
- Primary Citation of Related Structures:
8D2K, 8D2L, 8D2N, 8D2O, 8D2P, 8D2Q - PubMed Abstract:
Controlling the activity of the CRISPR-Cas9 system is essential to its safe adoption for clinical and research applications. Although the conformational dynamics of Cas9 are known to control its enzymatic activity, details of how Cas9 influences the catalytic processes at both nuclease domains remain elusive. Here we report five cryo-electron microscopy structures of the active Acidothermus cellulolyticus Cas9 complex along the reaction path at 2.2-2.9 Å resolution. We observed that a large movement in one nuclease domain, triggered by the cognate DNA, results in noticeable changes in the active site of the other domain that is required for metal coordination and catalysis. Furthermore, the conformations synchronize the reaction intermediates, enabling coupled cutting of the two DNA strands. Consistent with the roles of conformations in organizing the active sites, adjustments to the metal-coordination residues lead to altered metal specificity of A. cellulolyticus Cas9 and commonly used Streptococcus pyogenes Cas9 in cells.
- Institute of Molecular Biophysics, Florida State University, Tallahassee, FL, USA.
Organizational Affiliation: