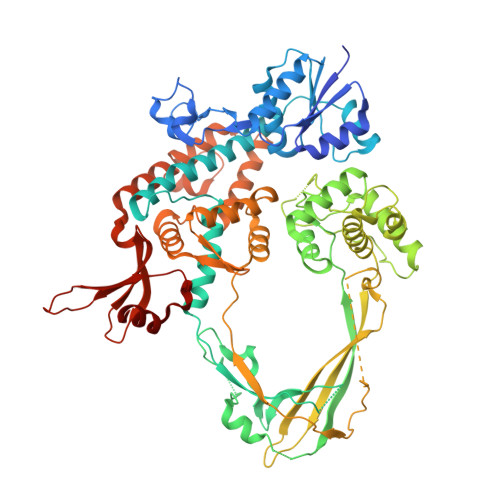
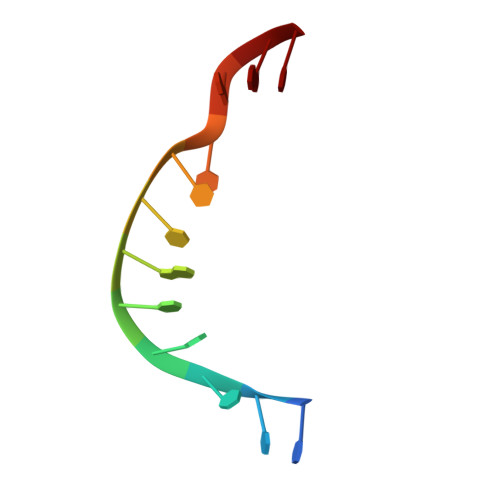
The interaction between transport-segment DNA and topoisomerase IA-crystal structure of MtbTOP1 in complex with both G- and T-segments.
Ferdous, S., Dasgupta, T., Annamalai, T., Tan, K., Tse-Dinh, Y.C.(2023) Nucleic Acids Res 51: 349-364
- PubMed: 36583363
- DOI: https://doi.org/10.1093/nar/gkac1205
- Primary Citation of Related Structures:
8CZQ - PubMed Abstract:
Each catalytic cycle of type IA topoisomerases has been proposed to comprise multistep reactions. The capture of the transport-segment DNA (T-segment) into the central cavity of the N-terminal toroidal structure is an important action, which is preceded by transient gate-segment (G-segment) cleavage and succeeded by G-segment religation for the relaxation of negatively supercoiled DNA and decatenation of DNA. The T-segment passage in and out of the central cavity requires significant domain-domain rearrangements, including the movement of D3 relative to D1 and D4 for the opening and closing of the gate towards the central cavity. Here we report a direct observation of the interaction of a duplex DNA in the central cavity of a type IA topoisomerase and its associated domain-domain conformational changes in a crystal structure of a Mycobacterium tuberculosis topoisomerase I complex that also has a bound G-segment. The duplex DNA within the central cavity illustrates the non-sequence-specific interplay between the T-segment DNA and the enzyme. The rich structural information revealed from the novel topoisomerase-DNA complex, in combination with targeted mutagenesis studies, provides new insights into the mechanism of the topoisomerase IA catalytic cycle.
- Department of Chemistry and Biochemistry, Florida International University, Miami, FL 33199, USA.
Organizational Affiliation: