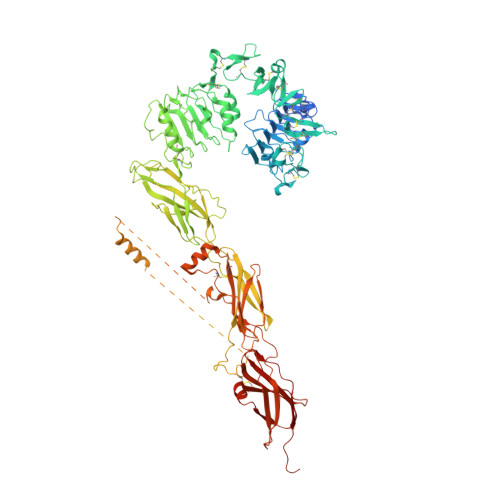
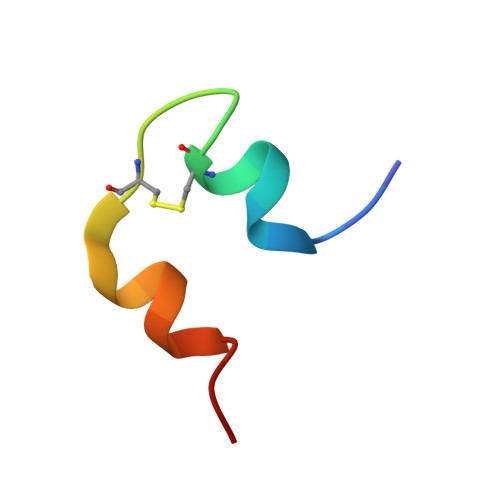
Structural conservation of insulin/IGF signalling axis at the insulin receptors level in Drosophila and humans.
Viola, C.M., Frittmann, O., Jenkins, H.T., Shafi, T., De Meyts, P., Brzozowski, A.M.(2023) Nat Commun 14: 6271-6271
- PubMed: 37805602
- DOI: https://doi.org/10.1038/s41467-023-41862-x
- Primary Citation of Related Structures:
8CLS - PubMed Abstract:
The insulin-related hormones regulate key life processes in Metazoa, from metabolism to growth, lifespan and aging, through an evolutionarily conserved insulin signalling axis (IIS). In humans the IIS axis is controlled by insulin, two insulin-like growth factors, two isoforms of the insulin receptor (hIR-A and -B), and its homologous IGF-1R. In Drosophila, this signalling engages seven insulin-like hormones (DILP1-7) and a single receptor (dmIR). This report describes the cryoEM structure of the dmIR ectodomain:DILP5 complex, revealing high structural homology between dmIR and hIR. The excess of DILP5 yields dmIR complex in an asymmetric 'T' conformation, similar to that observed in some complexes of human IRs. However, dmIR binds three DILP5 molecules in a distinct arrangement, showing also dmIR-specific features. This work adds structural support to evolutionary conservation of the IIS axis at the IR level, and also underpins a better understanding of an important model organism.
- York Structural Biology Laboratory, Department of Chemistry, University of York, Heslington, York, YO10 5DD, UK.
Organizational Affiliation: