CryoEM structure of the Aspergilus sp. fungal non-reducing polyketide synthase (NR-PKS) PksA at 2.6 Angstroms resolution
Munoz-Hernandez, H., Maier, T.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Norsolorinic acid synthase | 2,170 | Aspergillus parasiticus | Mutation(s): 0 Gene Names: aflC, pksA, pksL1, P875_00052995 EC: 2.3.1.221 | 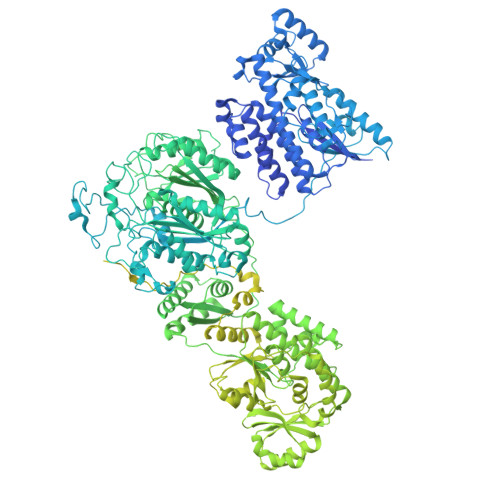 | |
UniProt | |||||
Find proteins for Q12053 (Aspergillus parasiticus (strain ATCC 56775 / NRRL 5862 / SRRC 143 / SU-1)) Explore Q12053 Go to UniProtKB: Q12053 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q12053 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | cryoSPARC | |
| MODEL REFINEMENT | PHENIX |
| Funding Organization | Location | Grant Number |
|---|---|---|
| European Commission | European Union | ID: 845941 |
| Swiss National Science Foundation | Switzerland | -- |