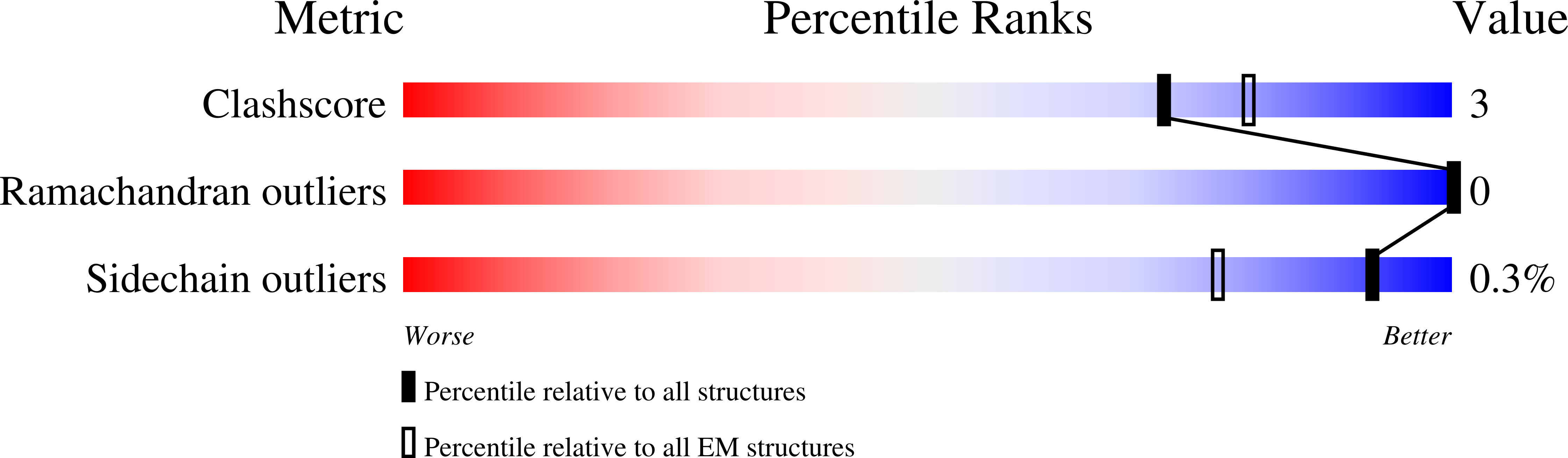Structure and function of Plasmodium actin II in the parasite mosquito stages.
Lopez, A.J., Andreadaki, M., Vahokoski, J., Deligianni, E., Calder, L.J., Camerini, S., Freitag, A., Bergmann, U., Rosenthal, P.B., Siden-Kiamos, I., Kursula, I.(2023) PLoS Pathog 19: e1011174-e1011174
- PubMed: 36877739
- DOI: https://doi.org/10.1371/journal.ppat.1011174
- Primary Citation of Related Structures:
8CCN, 8CCO - PubMed Abstract:
Actins are filament-forming, highly-conserved proteins in eukaryotes. They are involved in essential processes in the cytoplasm and also have nuclear functions. Malaria parasites (Plasmodium spp.) have two actin isoforms that differ from each other and from canonical actins in structure and filament-forming properties. Actin I has an essential role in motility and is fairly well characterized. The structure and function of actin II are not as well understood, but mutational analyses have revealed two essential functions in male gametogenesis and in the oocyst. Here, we present expression analysis, high-resolution filament structures, and biochemical characterization of Plasmodium actin II. We confirm expression in male gametocytes and zygotes and show that actin II is associated with the nucleus in both stages in filament-like structures. Unlike actin I, actin II readily forms long filaments in vitro, and near-atomic structures in the presence or absence of jasplakinolide reveal very similar structures. Small but significant differences compared to other actins in the openness and twist, the active site, the D-loop, and the plug region contribute to filament stability. The function of actin II was investigated through mutational analysis, suggesting that long and stable filaments are necessary for male gametogenesis, while a second function in the oocyst stage also requires fine-tuned regulation by methylation of histidine 73. Actin II polymerizes via the classical nucleation-elongation mechanism and has a critical concentration of ~0.1 μM at the steady-state, like actin I and canonical actins. Similarly to actin I, dimers are a stable form of actin II at equilibrium.
- Department of Biomedicine, University of Bergen, Bergen, Norway.
Organizational Affiliation: