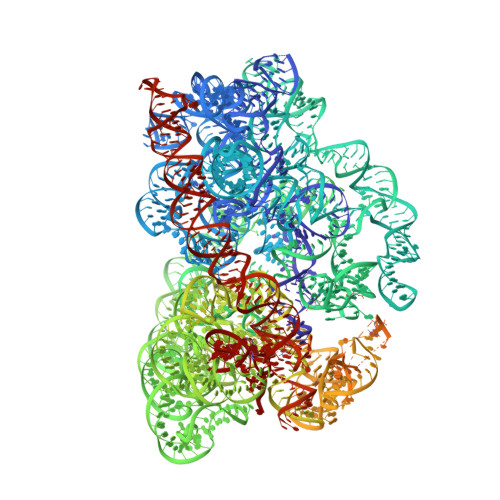
Structural conservation of antibiotic interaction with ribosomes.
Paternoga, H., Crowe-McAuliffe, C., Bock, L.V., Koller, T.O., Morici, M., Beckert, B., Myasnikov, A.G., Grubmuller, H., Novacek, J., Wilson, D.N.(2023) Nat Struct Mol Biol 30: 1380-1392
- PubMed: 37550453
- DOI: https://doi.org/10.1038/s41594-023-01047-y
- Primary Citation of Related Structures:
8CA7, 8CAI, 8CAM, 8CAZ, 8CEP, 8CEU, 8CF1, 8CF8, 8CGD, 8CGI, 8CGJ, 8CGK, 8CGR, 8CGU, 8CGV - PubMed Abstract:
The ribosome is a major target for clinically used antibiotics, but multidrug resistant pathogenic bacteria are making our current arsenal of antimicrobials obsolete. Here we present cryo-electron-microscopy structures of 17 distinct compounds from six different antibiotic classes bound to the bacterial ribosome at resolutions ranging from 1.6 to 2.2 Å. The improved resolution enables a precise description of antibiotic-ribosome interactions, encompassing solvent networks that mediate multiple additional interactions between the drugs and their target. Our results reveal a high structural conservation in the binding mode between antibiotics with the same scaffold, including ordered water molecules. Water molecules are visualized within the antibiotic binding sites that are preordered, become ordered in the presence of the drug and that are physically displaced on drug binding. Insight into RNA-ligand interactions will facilitate development of new antimicrobial agents, as well as other RNA-targeting therapies.
- Institute for Biochemistry and Molecular Biology, University of Hamburg, Hamburg, Germany.
Organizational Affiliation: