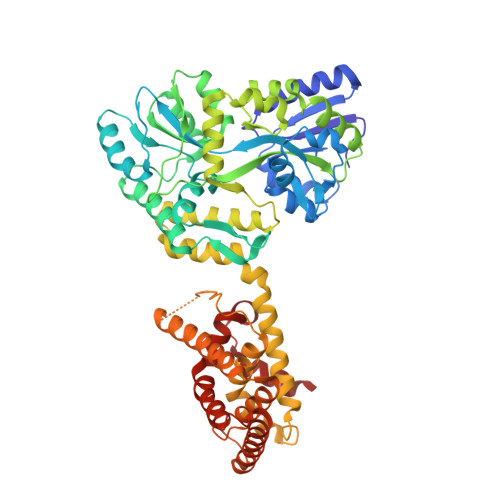
Structural Elucidation and Covalent Modulation of the Autorepressed Orphan Nuclear Receptor NR2F6.
Oerlemans, G.J.M., van den Oetelaar, M.C.M., van den Elzen, S.P., Brunsveld, L.(2025) ACS Chem Biol 20: 2308-2317
- PubMed: 40931005
- DOI: https://doi.org/10.1021/acschembio.5c00475
- Primary Citation of Related Structures:
8C5L - PubMed Abstract:
The orphan nuclear receptor NR2F6 (Nuclear Receptor subfamily 2 group F member 6) is an emerging therapeutic target for cancer immunotherapy. Upregulation of NR2F6 expression in tumor cells has been linked to proliferation and metastasis, while in immune cells NR2F6 inhibits antitumor T-cell responses. Small molecule modulation of NR2F6 activity might therefore be a novel strategy in cancer treatment, benefiting from this dual role of NR2F6. However, there are no molecular strategies available for targeting NR2F6, hampered among others by lack of structural insights and appropriate biochemical assays. To overcome these challenges, several noncanonical nuclear receptor coregulator peptide motifs were identified to be constitutively recruited to the NR2F6 ligand binding domain (LBD). Co-crystallization of the NR2F6 LBD with a peptide from the coregulator Nuclear Receptor Binding SET Domain Protein 1 (NSD1) enabled, for the first time, the structural elucidation of the unliganded (apo) form of NR2F6. This revealed an autorepressed, homodimeric LBD conformation in which helix 12 folds over the canonical coregulator binding site, generating an alternative contact surface for NSD1 binding. Screening of a focused library of covalent NR probes identified compounds that preferentially target a cysteine residue near the NSD1 binding site, inhibiting NR2F6 coregulator recruitment. Combined, these results provide structural insights into the ligand-independent transcriptional activity of NR2F6 and may serve as a starting point for the development of novel NR2F6 modulators.
- Laboratory of Chemical Biology, Department of Biomedical Engineering and Institute of Complex Molecular Systems, Technische Universiteit Eindhoven, 5612 AZ Eindhoven, The Netherlands.
Organizational Affiliation: