Recombinant Production, NMR Solution Structure, and Membrane Interaction of the Ph alpha 1 beta Toxin, a TRPA1 Modulator from the Brazilian Armed Spider Phoneutria nigriventer .
Lyukmanova, E.N., Mironov, P.A., Kulbatskii, D.S., Shulepko, M.A., Paramonov, A.S., Chernaya, E.M., Logashina, Y.A., Andreev, Y.A., Kirpichnikov, M.P., Shenkarev, Z.O.(2023) Toxins (Basel) 15
- PubMed: 37368679
- DOI: https://doi.org/10.3390/toxins15060378
- Primary Citation of Related Structures:
8BWB - PubMed Abstract:
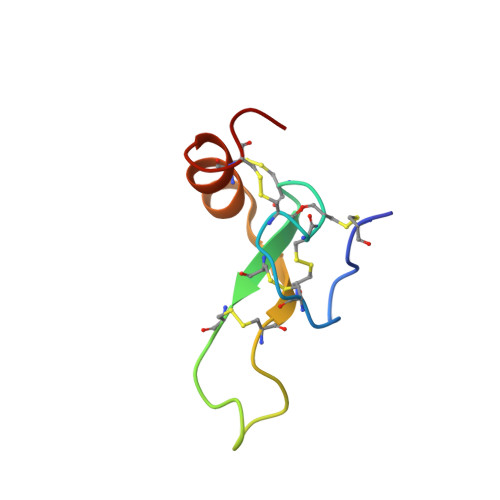
Phα1β (PnTx3-6) is a neurotoxin from the spider Phoneutria nigriventer venom, originally identified as an antagonist of two ion channels involved in nociception: N-type voltage-gated calcium channel (Ca V 2.2) and TRPA1. In animal models, Phα1β administration reduces both acute and chronic pain. Here, we report the efficient bacterial expression system for the recombinant production of Phα1β and its 15 N-labeled analogue. Spatial structure and dynamics of Phα1β were determined via NMR spectroscopy. The N -terminal domain (Ala1-Ala40) contains the inhibitor cystine knot (ICK or knottin) motif, which is common to spider neurotoxins. The C -terminal α-helix (Asn41-Cys52) stapled to ICK by two disulfides exhibits the µs-ms time-scale fluctuations. The Phα1β structure with the disulfide bond patterns Cys1-5, Cys2-7, Cys3-12, Cys4-10, Cys6-11, Cys8-9 is the first spider knottin with six disulfide bridges in one ICK domain, and is a good reference to other toxins from the ctenitoxin family. Phα1β has a large hydrophobic region on its surface and demonstrates a moderate affinity for partially anionic lipid vesicles at low salt conditions. Surprisingly, 10 µM Phα1β significantly increases the amplitude of diclofenac-evoked currents and does not affect the allyl isothiocyanate (AITC)-evoked currents through the rat TRPA1 channel expressed in Xenopus oocytes. Targeting several unrelated ion channels, membrane binding, and the modulation of TRPA1 channel activity allow for considering Phα1β as a gating modifier toxin, probably interacting with S1-S4 gating domains from a membrane-bound state.
- Department of Biology, MSU-BIT Shenzhen University, Shenzhen 518172, China.
Organizational Affiliation: