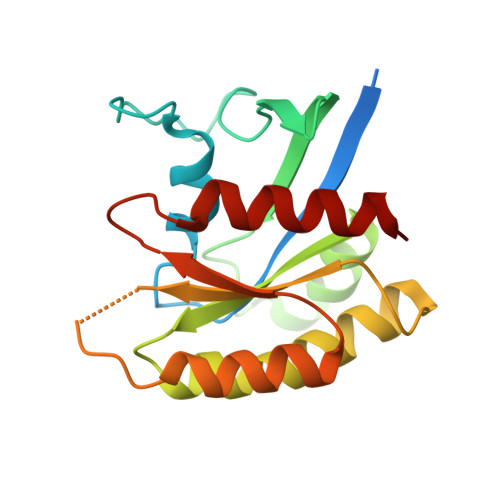
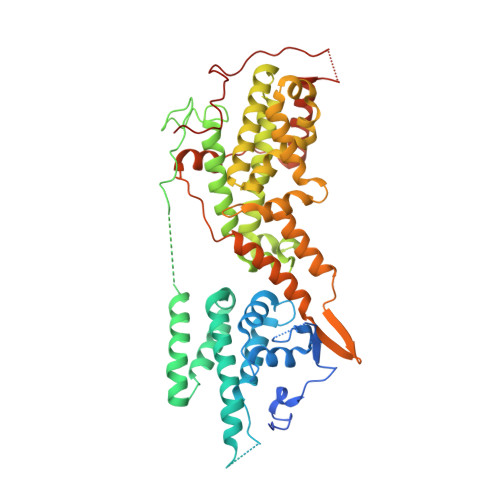
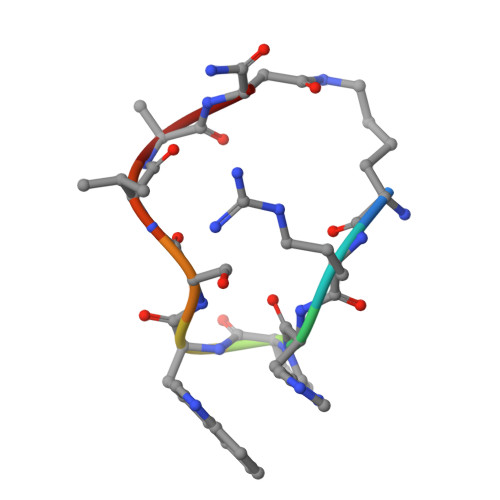
Nanobody Loop Mimetics Enhance Son of Sevenless 1-Catalyzed Nucleotide Exchange on RAS.
Van Holsbeeck, K., Fischer, B., Gonzalez, S., Gadais, C., Versees, W., Martins, J.C., Martin, C., Wohlkonig, A., Steyaert, J., Ballet, S.(2023) Angew Chem Int Ed Engl 62: e202219095-e202219095
- PubMed: 37067463
- DOI: https://doi.org/10.1002/anie.202219095
- Primary Citation of Related Structures:
8BE6, 8BE7, 8BE8, 8BE9, 8BEA - PubMed Abstract:
RAS proteins control various intracellular signaling networks. Mutations at specific locations were shown to stabilize their active guanosine triphosphate (GTP)-bound state, which is associated with the development of multiple cancers. An attractive approach to modulate RAS signaling is through its regulatory guanine nucleotide exchange factor (GEF) son of sevenless 1 (SOS1). With the recent discovery of Nanobody14 (Nb14), which potently enhances SOS1-catalyzed nucleotide exchange on RAS, we explored the feasibility of developing peptide mimetics by structurally mimicking the complementarity-determining region 3 (CDR3). Guided by a biochemical GEF assay and X-ray co-crystal structures, successive rounds of optimization and gradual conformational rigidification led to CDR3 mimetics showing half of the maximal activation potential of Nb14 with an EC 50 value of 29 μM. Altogether, this study demonstrated that peptides able to modulate a protein-protein interaction can be obtained by structural mimicry of a Nb paratope.
- Research Group of Organic Chemistry, Vrije Universiteit Brussel, Pleinlaan 2, 1050, Brussels, Belgium.
Organizational Affiliation: