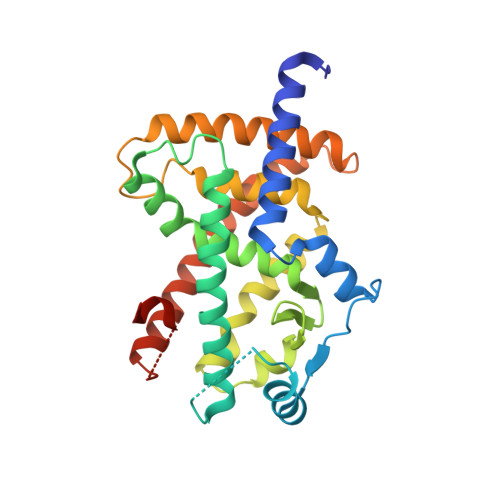
Discovery and characterization of orally bioavailable 4-chloro-6-fluoroisophthalamides as covalent PPARG inverse-agonists.
Orsi, D.L., Ferrara, S.J., Siegel, S., Friberg, A., Bouche, L., Pook, E., Lienau, P., Bluck, J.P., Lemke, C.T., Akcay, G., Stellfeld, T., Meyer, H., Putter, V., Holton, S.J., Korr, D., Jerchel-Furau, I., Pantelidou, C., Strathdee, C.A., Meyerson, M., Eis, K., Goldstein, J.T.(2022) Bioorg Med Chem 78: 117130-117130
- PubMed: 36542958
- DOI: https://doi.org/10.1016/j.bmc.2022.117130
- Primary Citation of Related Structures:
8B8W, 8B8X, 8B8Y, 8B8Z, 8B90, 8B91, 8B92, 8B93, 8B94, 8B95 - PubMed Abstract:
PPAR gamma (PPARG) is a ligand activated transcription factor that regulates genes involved in inflammation, bone biology, lipid homeostasis, as well as a master regulator of adipogenesis and a potential lineage driver of luminal bladder cancer. While PPARG agonists lead to transcriptional activation of canonical target genes, inverse agonists have the opposite effect through inducing a transcriptionally repressive complex leading to repression of canonical target gene expression. While many agonists have been described and tested clinically, inverse agonists offer an underexplored avenue to modulate PPARG biology in vivo. Current inverse agonists lack favorable in vivo properties; herein we describe the discovery and characterization of a series of orally bioavailable 4-chloro-6-fluoroisophthalamides as covalent PPARG inverse-agonists, BAY-5516, BAY-5094, and BAY-9683. Structural studies of this series revealed distinct pre- and post-covalent binding positions, which led to the hypothesis that interactions in the pre-covalent conformation are primarily responsible for driving affinity, while interactions in the post-covalent conformation are more responsible for cellular functional effects by enhancing PPARG interactions with its corepressors. The need to simultaneously optimize for two distinct states may partially explain the steep SAR observed. Exquisite selectivity was achieved over related nuclear receptors in the subfamily due in part to a covalent warhead with low reactivity through an S N Ar mechanism in addition to the specificity gained through covalent binding to a reactive cysteine uniquely positioned within the PPARG LBD. BAY-5516, BAY-5094, and BAY-9683 lead to pharmacodynamic regulation of PPARG target gene expression in vivo comparable to known inverse agonist SR10221 and represent new tools for future in vivo studies to explore their potential utility for treatment of disorders of hyperactivated PPARG including luminal bladder cancer and other disorders.
- Center for the Development of Therapeutics, Broad Institute of MIT and Harvard, Cambridge, MA 02142, USA.
Organizational Affiliation: