Structural basis of mitochondrial membrane bending by the I-II-III 2 -IV 2 supercomplex.
Muhleip, A., Flygaard, R.K., Baradaran, R., Haapanen, O., Gruhl, T., Tobiasson, V., Marechal, A., Sharma, V., Amunts, A.(2023) Nature 615: 934-938
- PubMed: 36949187
- DOI: https://doi.org/10.1038/s41586-023-05817-y
- Primary Citation of Related Structures:
8B6F, 8B6G, 8B6H, 8B6J, 8BQS - PubMed Abstract:
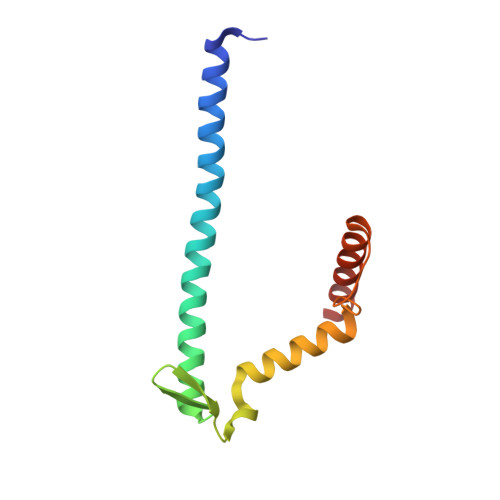
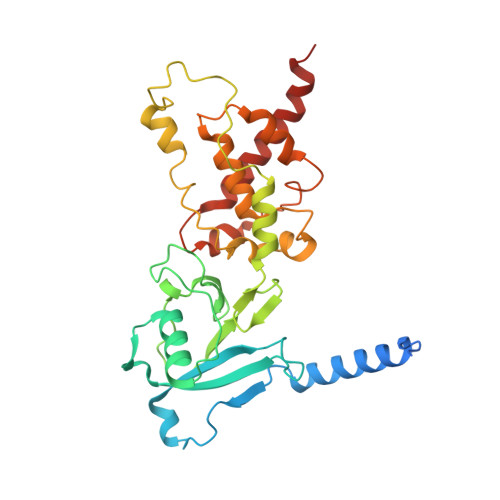
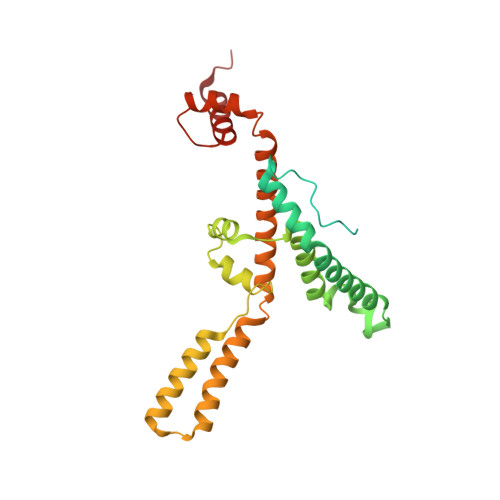
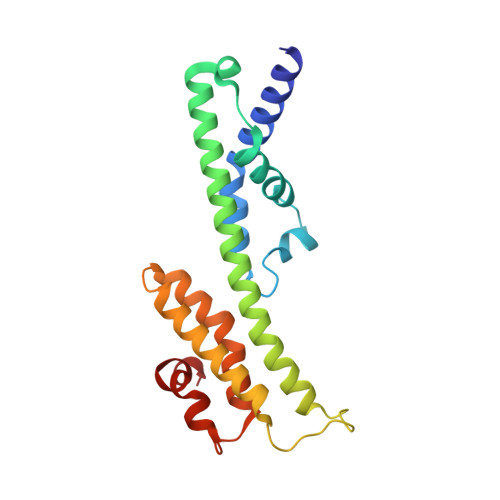
Mitochondrial energy conversion requires an intricate architecture of the inner mitochondrial membrane 1 . Here we show that a supercomplex containing all four respiratory chain components contributes to membrane curvature induction in ciliates. We report cryo-electron microscopy and cryo-tomography structures of the supercomplex that comprises 150 different proteins and 311 bound lipids, forming a stable 5.8-MDa assembly. Owing to subunit acquisition and extension, complex I associates with a complex IV dimer, generating a wedge-shaped gap that serves as a binding site for complex II. Together with a tilted complex III dimer association, it results in a curved membrane region. Using molecular dynamics simulations, we demonstrate that the divergent supercomplex actively contributes to the membrane curvature induction and tubulation of cristae. Our findings highlight how the evolution of protein subunits of respiratory complexes has led to the I-II-III 2 -IV 2 supercomplex that contributes to the shaping of the bioenergetic membrane, thereby enabling its functional specialization.
- Science for Life Laboratory, Department of Biochemistry and Biophysics, Stockholm University, Solna, Sweden.
Organizational Affiliation: