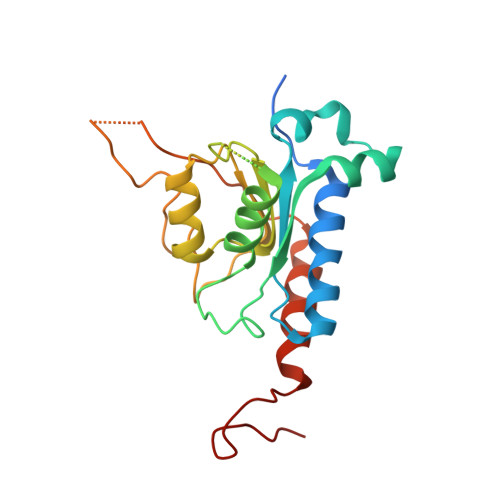
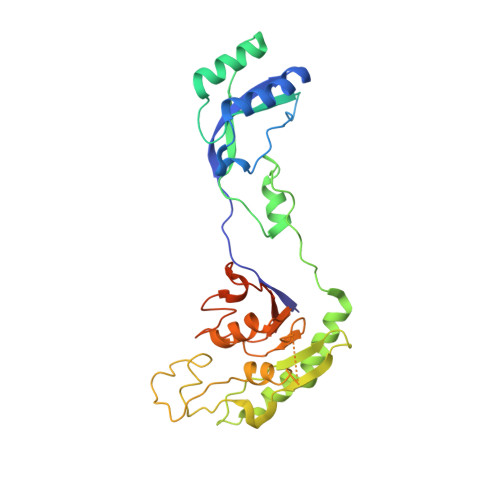
Structural basis for sequence-independent substrate selection by eukaryotic wobble base tRNA deaminase ADAT2/3.
Dolce, L.G., Zimmer, A.A., Tengo, L., Weis, F., Rubio, M.A.T., Alfonzo, J.D., Kowalinski, E.(2022) Nat Commun 13: 6737-6737
- PubMed: 36347890
- DOI: https://doi.org/10.1038/s41467-022-34441-z
- Primary Citation of Related Structures:
8AW3 - PubMed Abstract:
The essential deamination of adenosine A 34 to inosine at the wobble base is the individual tRNA modification with the greatest effects on mRNA decoding, empowering a single tRNA to translate three different codons. To date, many aspects of how eukaryotic deaminases specifically select their multiple substrates remain unclear. Here, using cryo-EM, we present the structure of a eukaryotic ADAT2/3 deaminase bound to a full-length tRNA, revealing that the enzyme distorts the anticodon loop, but in contrast to the bacterial enzymes, selects its substrate via sequence-independent contacts of eukaryote-acquired flexible or intrinsically unfolded motifs distal from the conserved catalytic core. A gating mechanism for substrate entry to the active site is identified. Our multi-step tRNA recognition model yields insights into how RNA editing by A 34 deamination evolved, shaped the genetic code, and directly impacts the eukaryotic proteome.
- EMBL Grenoble, 71 Avenue des Martyrs, 38042, Grenoble, France.
Organizational Affiliation: