X-ray structure of Na+-NQR from Vibrio cholerae at 3.4 A resolution
Fritz, G.(2023) Nat Struct Mol Biol
Experimental Data Snapshot
Starting Model: experimental
View more details
(2023) Nat Struct Mol Biol
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Na(+)-translocating NADH-quinone reductase subunit A | 468 | Vibrio cholerae | Mutation(s): 0 Gene Names: nqrA, ERS013165_00619, ERS013186_02081, ERS013199_02394, ERS013202_01882, ERS013206_02986, ERS013207_01957 EC: 7.2.1.1 | 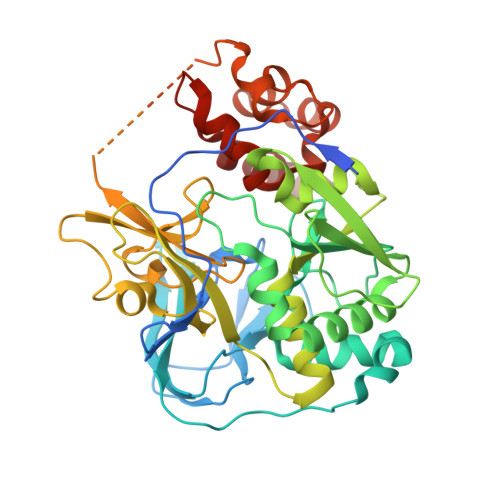 | |
UniProt | |||||
Find proteins for A5F5X1 (Vibrio cholerae serotype O1 (strain ATCC 39541 / Classical Ogawa 395 / O395)) Explore A5F5X1 Go to UniProtKB: A5F5X1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A5F5X1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Na(+)-translocating NADH-quinone reductase subunit B | 415 | Vibrio cholerae | Mutation(s): 0 Gene Names: nqrB, D6U24_04465, ERS013186_02082, ERS013198_02508, ERS013199_02395, ERS013200_04117, ERS013202_01883, ERS013206_02987, ERS013207_01958, EYB64_17950... EC: 7.2.1.1 Membrane Entity: Yes | 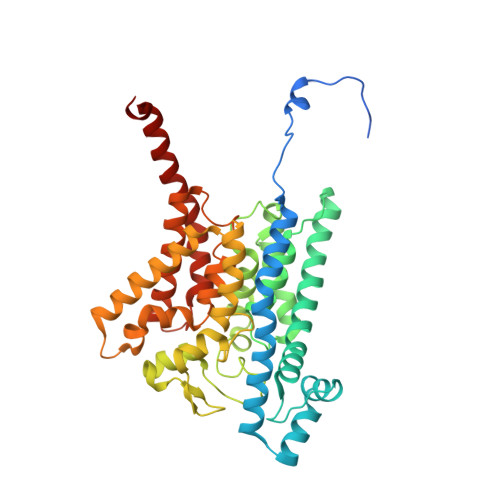 | |
UniProt | |||||
Find proteins for A5F5X0 (Vibrio cholerae serotype O1 (strain ATCC 39541 / Classical Ogawa 395 / O395)) Explore A5F5X0 Go to UniProtKB: A5F5X0 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A5F5X0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Na(+)-translocating NADH-quinone reductase subunit C | 257 | Vibrio cholerae | Mutation(s): 0 Gene Names: nqrC, BC353_01370, D6U24_04470, ERS013165_00616, ERS013186_02083, ERS013198_02507, ERS013199_02396, ERS013200_04118, ERS013201_01110, ERS013202_01884... EC: 7.2.1.1 Membrane Entity: Yes | 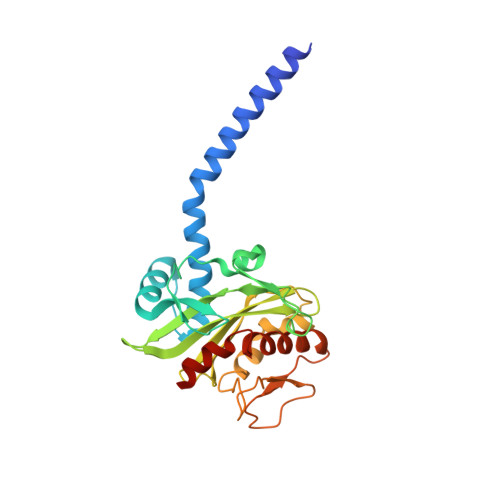 | |
UniProt | |||||
Find proteins for A5F5Y7 (Vibrio cholerae serotype O1 (strain ATCC 39541 / Classical Ogawa 395 / O395)) Explore A5F5Y7 Go to UniProtKB: A5F5Y7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A5F5Y7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Na(+)-translocating NADH-quinone reductase subunit D | 210 | Vibrio cholerae | Mutation(s): 0 Gene Names: nqrD, BC353_01365, D6U24_04475, ERS013165_00615, ERS013186_02084, ERS013198_02506, ERS013199_02397, ERS013200_04119, ERS013201_01111, ERS013202_01885... EC: 7.2.1.1 Membrane Entity: Yes | 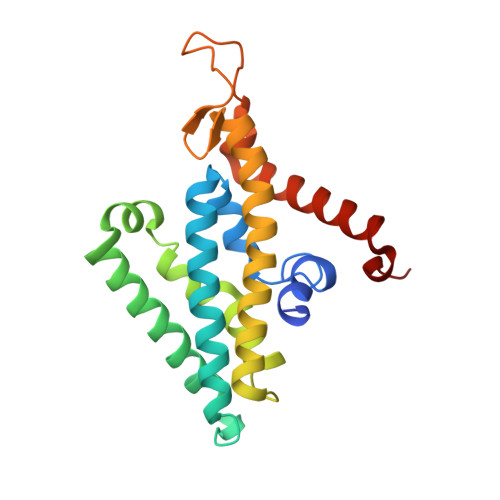 | |
UniProt | |||||
Find proteins for A5F5Y6 (Vibrio cholerae serotype O1 (strain ATCC 39541 / Classical Ogawa 395 / O395)) Explore A5F5Y6 Go to UniProtKB: A5F5Y6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A5F5Y6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 5 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Na(+)-translocating NADH-quinone reductase subunit E | 198 | Vibrio cholerae | Mutation(s): 0 Gene Names: nqrE, BC353_01360, D6U24_04480, ERS013165_00614, ERS013186_02085, ERS013198_02505, ERS013199_02398, ERS013200_04120, ERS013201_01112, ERS013202_01886... EC: 7.2.1.1 Membrane Entity: Yes |  | |
UniProt | |||||
Find proteins for A5F5Y5 (Vibrio cholerae serotype O1 (strain ATCC 39541 / Classical Ogawa 395 / O395)) Explore A5F5Y5 Go to UniProtKB: A5F5Y5 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A5F5Y5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 6 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Na(+)-translocating NADH-quinone reductase subunit F | 408 | Vibrio cholerae | Mutation(s): 0 Gene Names: nqrF, D6U24_04485, ERS013198_02504, ERS013199_02399, ERS013201_01113, ERS013202_01887, ERS013206_02991, EYB64_17930, FLM12_12900 EC: 7.2.1.1 Membrane Entity: Yes |  | |
UniProt | |||||
Find proteins for A5F5Y4 (Vibrio cholerae serotype O1 (strain ATCC 39541 / Classical Ogawa 395 / O395)) Explore A5F5Y4 Go to UniProtKB: A5F5Y4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A5F5Y4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 9 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| FAD Query on FAD | R [auth F] | FLAVIN-ADENINE DINUCLEOTIDE C27 H33 N9 O15 P2 VWWQXMAJTJZDQX-UYBVJOGSSA-N |  | ||
| 3PE Query on 3PE | J [auth B] | 1,2-Distearoyl-sn-glycerophosphoethanolamine C41 H82 N O8 P LVNGJLRDBYCPGB-LDLOPFEMSA-N |  | ||
| LMT Query on LMT | I [auth B], O [auth D], Q [auth E] | DODECYL-BETA-D-MALTOSIDE C24 H46 O11 NLEBIOOXCVAHBD-QKMCSOCLSA-N |  | ||
| FMN Query on FMN | G [auth B], M [auth C] | FLAVIN MONONUCLEOTIDE C17 H21 N4 O9 P FVTCRASFADXXNN-SCRDCRAPSA-N |  | ||
| RBF Query on RBF | H [auth B] | RIBOFLAVIN C17 H20 N4 O6 AUNGANRZJHBGPY-SCRDCRAPSA-N |  | ||
| FES Query on FES | P [auth D], S [auth F] | FE2/S2 (INORGANIC) CLUSTER Fe2 S2 NIXDOXVAJZFRNF-UHFFFAOYSA-N |  | ||
| BR Query on BR | N [auth C] | BROMIDE ION Br CPELXLSAUQHCOX-UHFFFAOYSA-M |  | ||
| K Query on K | L [auth B] | POTASSIUM ION K NPYPAHLBTDXSSS-UHFFFAOYSA-N |  | ||
| NA Query on NA | K [auth B] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 89.31 | α = 90 |
| b = 142.15 | β = 109.83 |
| c = 105.93 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XSCALE | data scaling |
| PDB_EXTRACT | data extraction |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| German Research Foundation (DFG) | Germany | 311211092 |