Avidin + Biotin-Tempo
Milani, J., Lau, K., Pojer, F., Ansermet, J.P., Saenz, F.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Avidin | 152 | Gallus gallus | Mutation(s): 0 | 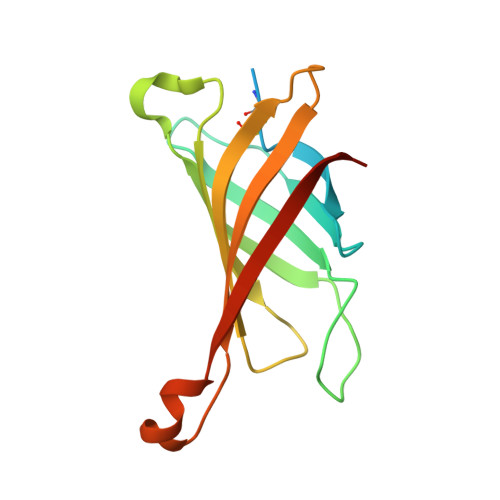 | |
UniProt | |||||
Find proteins for P02701 (Gallus gallus) Explore P02701 Go to UniProtKB: P02701 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P02701 | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | |||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| KFL (Subject of Investigation/LOI) Query on KFL | G [auth A], L [auth B], N [auth C], S [auth D] | 6-[(3~{a}~{S},4~{S},6~{a}~{R})-2-oxidanylidene-1,3,3~{a},4,6,6~{a}-hexahydrothieno[3,4-d]imidazol-4-yl]-~{N}-(2,2,6,6-tetramethyl-1-oxidanyl-piperidin-4-yl)hexanamide C20 H36 N4 O3 S PUXBASNOQZMIKI-ZOBUZTSGSA-N |  | ||
| NAG Query on NAG | F [auth A], K [auth B], R [auth D] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| ZN Query on ZN | H [auth A], I [auth A], O [auth C], P [auth C] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| NA Query on NA | J [auth A], M [auth B], Q [auth C], T [auth D] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 59.353 | α = 90 |
| b = 80.952 | β = 119.079 |
| c = 60.951 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XDS | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Other government | -- |