The structural landscape and diversity of Pyricularia oryzae MAX effectors revisited.
Lahfa, M., Barthe, P., de Guillen, K., Cesari, S., Raji, M., Kroj, T., Le Naour-Vernet, M., Hoh, F., Gladieux, P., Roumestand, C., Gracy, J., Declerck, N., Padilla, A.(2024) PLoS Pathog 20: e1012176-e1012176
- PubMed: 38709846
- DOI: https://doi.org/10.1371/journal.ppat.1012176
- Primary Citation of Related Structures:
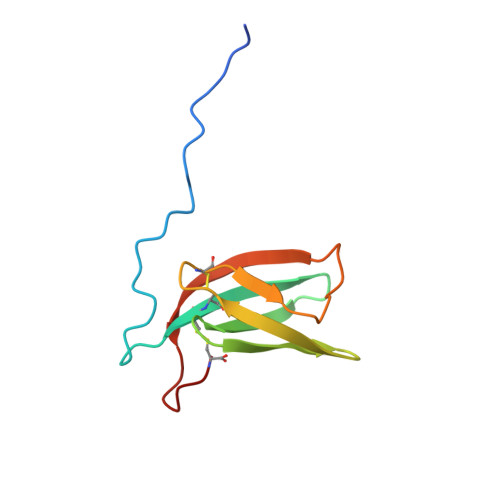
7ZJY, 7ZK0, 7ZKD, 8C8A - PubMed Abstract:
Magnaporthe AVRs and ToxB-like (MAX) effectors constitute a family of secreted virulence proteins in the fungus Pyricularia oryzae (syn. Magnaporthe oryzae), which causes blast disease on numerous cereals and grasses. In spite of high sequence divergence, MAX effectors share a common fold characterized by a ß-sandwich core stabilized by a conserved disulfide bond. In this study, we investigated the structural landscape and diversity within the MAX effector repertoire of P. oryzae. Combining experimental protein structure determination and in silico structure modeling we validated the presence of the conserved MAX effector core domain in 77 out of 94 groups of orthologs (OG) identified in a previous population genomic study. Four novel MAX effector structures determined by NMR were in remarkably good agreement with AlphaFold2 (AF2) predictions. Based on the comparison of the AF2-generated 3D models we propose a classification of the MAX effectors superfamily in 20 structural groups that vary in the canonical MAX fold, disulfide bond patterns, and additional secondary structures in N- and C-terminal extensions. About one-third of the MAX family members remain singletons, without strong structural relationship to other MAX effectors. Analysis of the surface properties of the AF2 MAX models also highlights the high variability within the MAX family at the structural level, potentially reflecting the wide diversity of their virulence functions and host targets.
- Centre de Biologie Structurale, Univ Montpellier, CNRS UMR 5048, INSERM U 1054, Montpellier, France.
Organizational Affiliation: