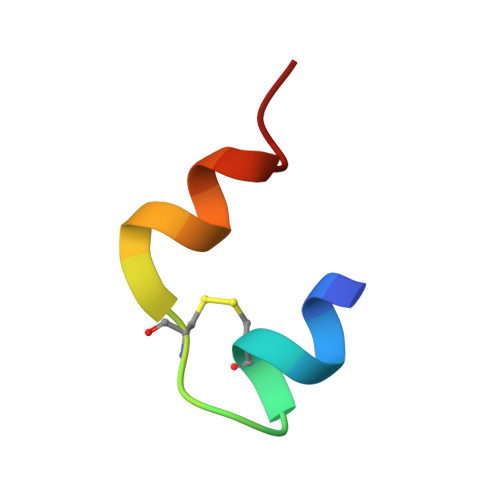
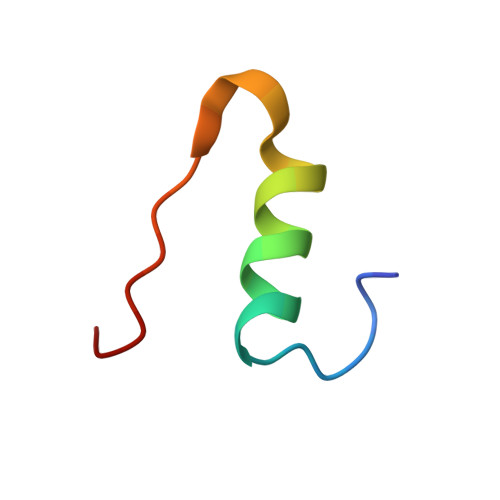
Dissociation of Hexameric Insulin Facilitated by Macrophage Migration Inhibitory Factor Indicates a Novel Role in Insulin Signalling
Wahid, A.A., Kasaar, O., Ronsse, D., Smith, K., Hyland, A., Staddon, W., Scrivens, B., Babarinde, S.O., Dunphy, R., Cozier, G.E., Koumanov, F., Crennell, S.J., Williams, R.J., van den Elsen, J.M.H.To be published.