"Redirecting an anti-IL-1 beta antibody to bind a new, unrelated and computationally predicted epitope on hIL-17A".
Fischman, S., Levin, I., Rondeau, J.M., Strajbl, M., Lehmann, S., Huber, T., Nimrod, G., Cebe, R., Omer, D., Kovarik, J., Bernstein, S., Sasson, Y., Demishtein, A., Shlamkovich, T., Bluvshtein, O., Grossman, N., Barak-Fuchs, R., Zhenin, M., Fastman, Y., Twito, S., Vana, T., Zur, N., Ofran, Y.(2023) Commun Biol 6: 997-997
- PubMed: 37773269
- DOI: https://doi.org/10.1038/s42003-023-05369-x
- Primary Citation of Related Structures:
7Z2M, 7Z3W, 7Z4T - PubMed Abstract:
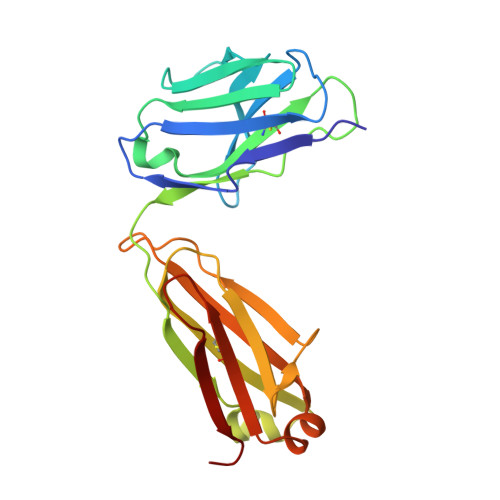
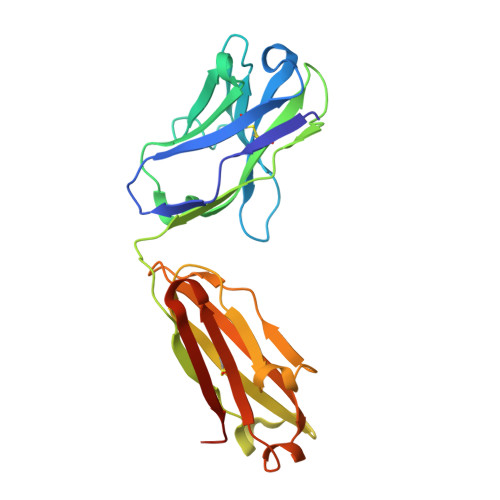
Antibody engineering technology is at the forefront of therapeutic antibody development. The primary goal for engineering a therapeutic antibody is the generation of an antibody with a desired specificity, affinity, function, and developability profile. Mature antibodies are considered antigen specific, which may preclude their use as a starting point for antibody engineering. Here, we explore the plasticity of mature antibodies by engineering novel specificity and function to a pre-selected antibody template. Using a small, focused library, we engineered AAL160, an anti-IL-1β antibody, to bind the unrelated antigen IL-17A, with the introduction of seven mutations. The final redesigned antibody, 11.003, retains favorable biophysical properties, binds IL-17A with sub-nanomolar affinity, inhibits IL-17A binding to its cognate receptor and is functional in a cell-based assay. The epitope of the engineered antibody can be computationally predicted based on the sequence of the template antibody, as is confirmed by the crystal structure of the 11.003/IL-17A complex. The structures of the 11.003/IL-17A and the AAL160/IL-1β complexes highlight the contribution of germline residues to the paratopes of both the template and re-designed antibody. This case study suggests that the inherent plasticity of antibodies allows for re-engineering of mature antibodies to new targets, while maintaining desirable developability profiles.
- Biolojic Design LTD, Rehovot, Israel.
Organizational Affiliation: