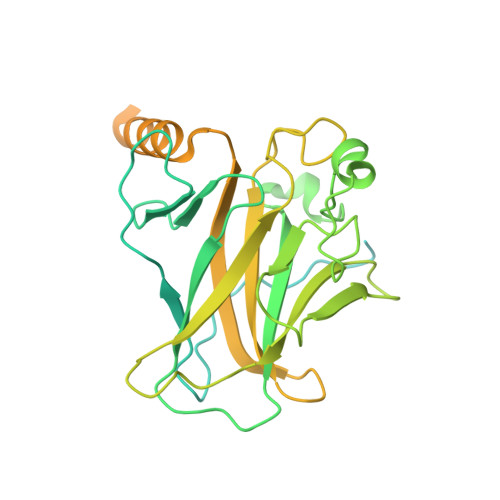
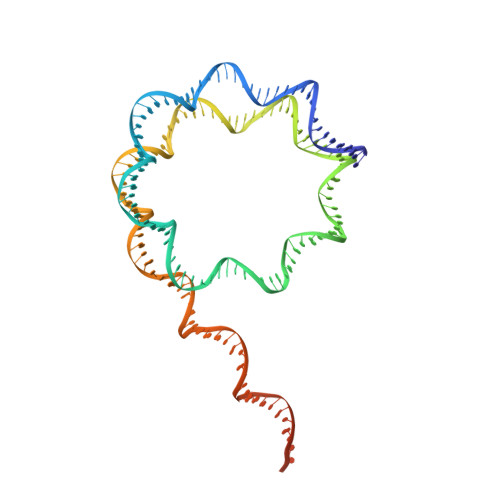
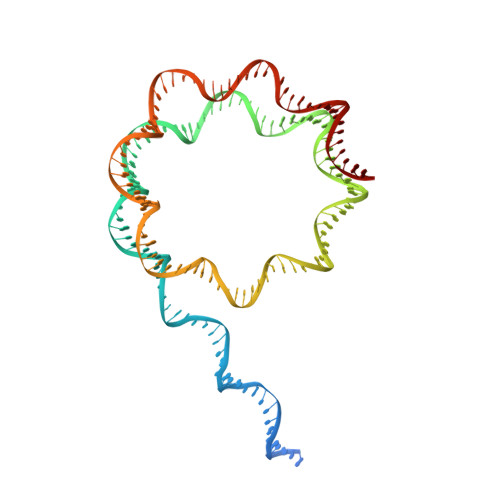
Structural basis for p53 binding to its nucleosomal target DNA sequence.
Nishimura, M., Takizawa, Y., Nozawa, K., Kurumizaka, H.(2022) PNAS Nexus 1: pgac177-pgac177
- PubMed: 36714865
- DOI: https://doi.org/10.1093/pnasnexus/pgac177
- Primary Citation of Related Structures:
7XZX, 7XZY, 7XZZ, 7Y00 - PubMed Abstract:
The tumor suppressor p53 functions as a pioneer transcription factor that binds a nucleosomal target DNA sequence. However, the mechanism by which p53 binds to its target DNA in the nucleosome remains elusive. Here we report the cryo-electron microscopy structures of the p53 DNA-binding domain and the full-length p53 protein complexed with a nucleosome containing the 20 base-pair target DNA sequence of p53 (p53BS). In the p53-nucleosome structures, the p53 DNA-binding domain forms a tetramer and specifically binds to the p53BS DNA, located near the entry/exit region of the nucleosome. The nucleosomal position of the p53BS DNA is within the genomic p21 promoter region. The p53 binding peels the DNA from the histone surface, and drastically changes the DNA path around the p53BS on the nucleosome. The C-terminal domain of p53 also binds to the DNA around the center and linker DNA regions of the nucleosome, as revealed by hydroxyl radical footprinting. These results provide important structural information for understanding the mechanism by which p53 binds the nucleosome and changes the chromatin structure for gene activation.
- Laboratory of Chromatin Structure and Function, Institute for Quantitative Biosciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-0032, Japan.
Organizational Affiliation: