Structural insights into cyanobacterial RuBisCO assembly coordinated by two chaperones Raf1 and RbcX.
Li, Q., Jiang, Y.L., Xia, L.Y., Chen, Y., Zhou, C.Z.(2022) Cell Discov 8: 93-93
- PubMed: 36123352
- DOI: https://doi.org/10.1038/s41421-022-00436-9
- Primary Citation of Related Structures:
7XSD - PubMed Abstract:
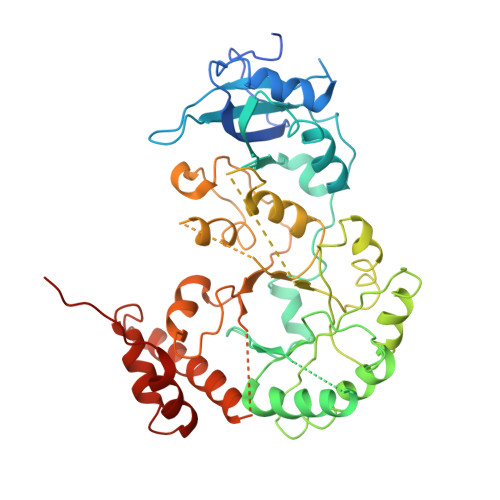
RuBisCO is the most abundant enzyme in nature, catalyzing the fixation of CO 2 in photosynthesis. Its common form consists of eight RbcL and eight RbcS subunits, the assembly of which requires a series of chaperones that include RbcX and RuBisCO accumulation factor 1 (Raf1). To understand how these RuBisCO-specific chaperones function during cyanobacterial RbcL 8 RbcS 8 (L 8 S 8 ) holoenzyme formation, we solved a 3.3-Å cryo-electron microscopy structure of a 32-subunit RbcL 8 Raf1 8 RbcX 16 (L 8 F 8 X 16 ) assembly intermediate from Anabaena sp. PCC 7120. Comparison to the previously resolved L 8 F 8 and L 8 X 16 structures together with biochemical assays revealed that the L 8 F 8 X 16 complex forms a rather dynamic structural intermediate, favoring RbcS displacement of Raf1 and RbcX. In vitro assays further demonstrated that both Raf1 and RbcX function to regulate RuBisCO condensate formation by restricting CcmM35 binding to the stably assembled L 8 S 8 holoenzymes. Combined with previous findings, we propose a model on how Raf1 and RbcX work in concert to facilitate, and regulate, cyanobacterial RuBisCO assembly as well as disassembly of RuBisCO condensates.
- School of Life Sciences, University of Science and Technology of China, Hefei, Anhui, China.
Organizational Affiliation: