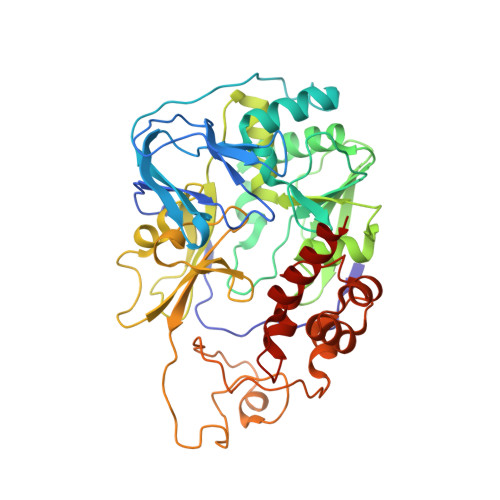
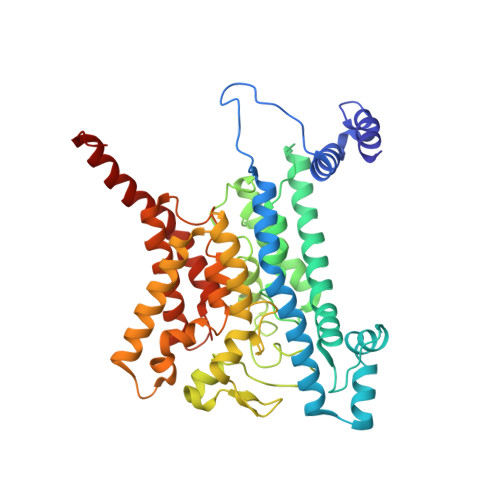
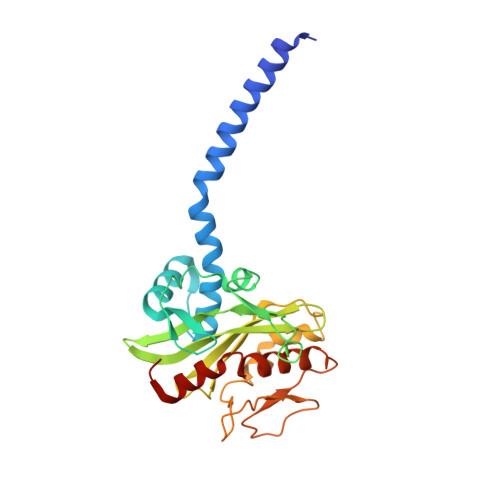
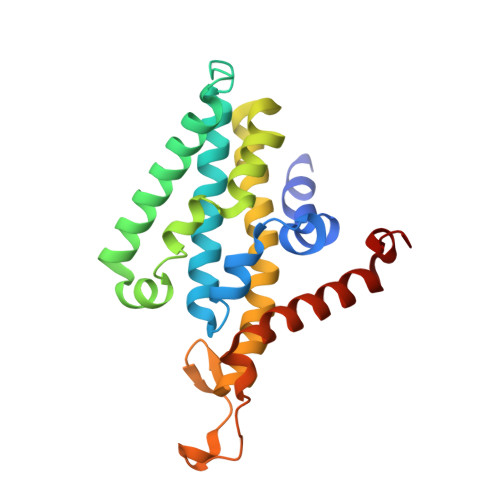
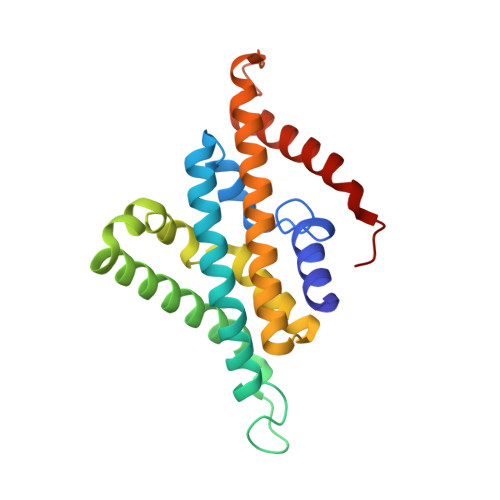
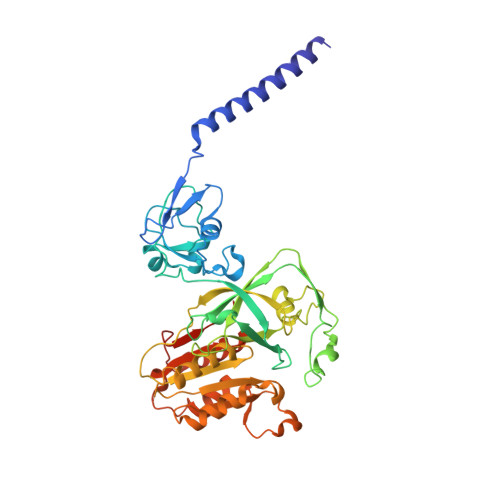
Cryo-EM structures of Na + -pumping NADH-ubiquinone oxidoreductase from Vibrio cholerae.
Kishikawa, J.I., Ishikawa, M., Masuya, T., Murai, M., Kitazumi, Y., Butler, N.L., Kato, T., Barquera, B., Miyoshi, H.(2022) Nat Commun 13: 4082-4082
- PubMed: 35882843
- DOI: https://doi.org/10.1038/s41467-022-31718-1
- Primary Citation of Related Structures:
7XK3, 7XK4, 7XK5, 7XK6, 7XK7 - PubMed Abstract:
The Na + -pumping NADH-ubiquinone oxidoreductase (Na + -NQR) couples electron transfer from NADH to ubiquinone with Na + -pumping, generating an electrochemical Na + gradient that is essential for energy-consuming reactions in bacteria. Since Na + -NQR is exclusively found in prokaryotes, it is a promising target for highly selective antibiotics. However, the molecular mechanism of inhibition is not well-understood for lack of the atomic structural information about an inhibitor-bound state. Here we present cryo-electron microscopy structures of Na + -NQR from Vibrio cholerae with or without a bound inhibitor at 2.5- to 3.1-Å resolution. The structures reveal the arrangement of all six redox cofactors including a herein identified 2Fe-2S cluster located between the NqrD and NqrE subunits. A large part of the hydrophilic NqrF is barely visible in the density map, suggesting a high degree of flexibility. This flexibility may be responsible to reducing the long distance between the 2Fe-2S centers in NqrF and NqrD/E. Two different types of specific inhibitors bind to the N-terminal region of NqrB, which is disordered in the absence of inhibitors. The present study provides a foundation for understanding the function of Na + -NQR and the binding manner of specific inhibitors.
- Institute for Protein Research, Osaka University, 3-2 Yamadaoka, Suita, Osaka, 565-0871, Japan.
Organizational Affiliation: