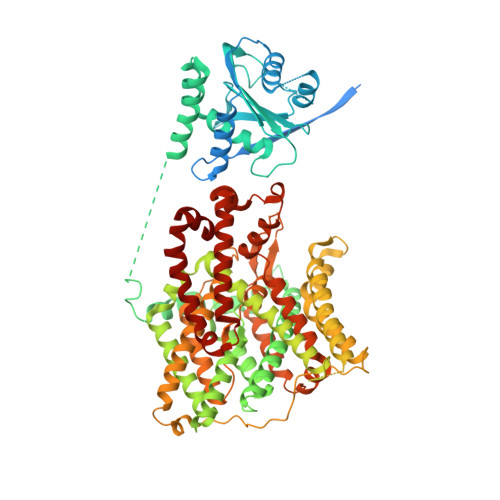
Structural insights into the conformational changes of BTR1/SLC4A11 in complex with PIP 2.
Lu, Y., Zuo, P., Chen, H., Shan, H., Wang, W., Dai, Z., Xu, H., Chen, Y., Liang, L., Ding, D., Jin, Y., Yin, Y.(2023) Nat Commun 14: 6157-6157
- PubMed: 37788993
- DOI: https://doi.org/10.1038/s41467-023-41924-0
- Primary Citation of Related Structures:
7X1G, 7X1H, 7X1I, 7X1J - PubMed Abstract:
BTR1 (SLC4A11) is a NH 3 stimulated H + (OH - ) transporter belonging to the SLC4 family. Dysfunction of BTR1 leads to diseases such as congenital hereditary endothelial dystrophy (CHED) and Fuchs endothelial corneal dystrophy (FECD). However, the mechanistic basis of BTR1 activation by alkaline pH, transport activity regulation and pathogenic mutations remains elusive. Here, we present cryo-EM structures of human BTR1 in the outward-facing state in complex with its activating ligands PIP 2 and the inward-facing state with the pathogenic R125H mutation. We reveal that PIP 2 binds at the interface between the transmembrane domain and the N-terminal cytosolic domain of BTR1. Disruption of either the PIP 2 binding site or protonation of PIP 2 phosphate groups by acidic pH can transform BTR1 into an inward-facing conformation. Our results provide insights into the mechanisms of how the transport activity and conformation changes of BTR1 are regulated by PIP 2 binding and interaction of TMD and NTD.
- Institute of Precision Medicine, Peking University Shenzhen Hospital, Shenzhen, 518036, China.
Organizational Affiliation: