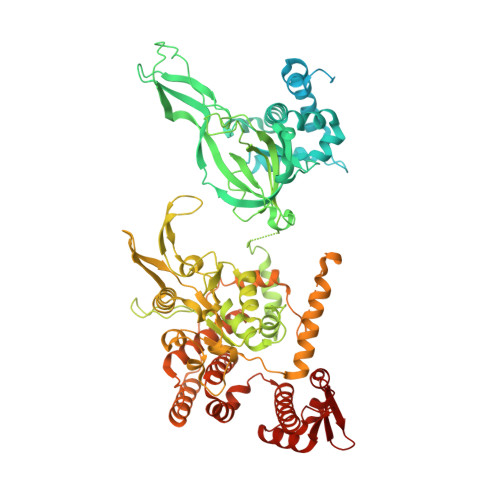
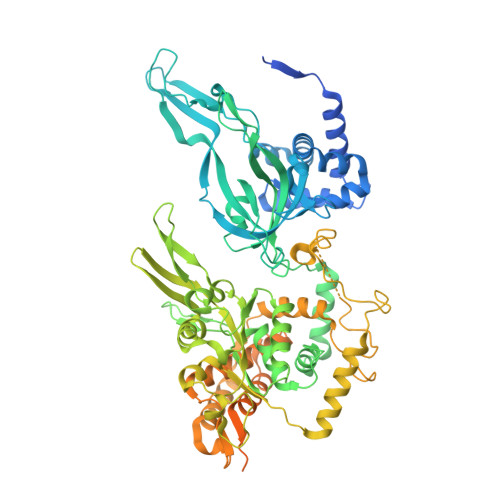
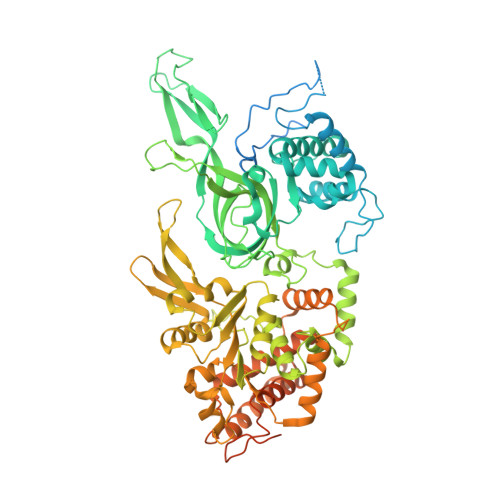
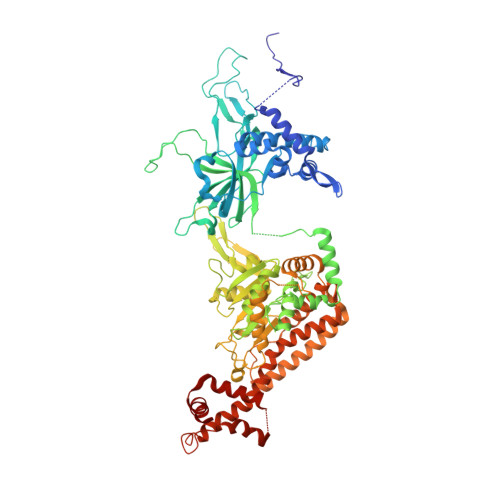
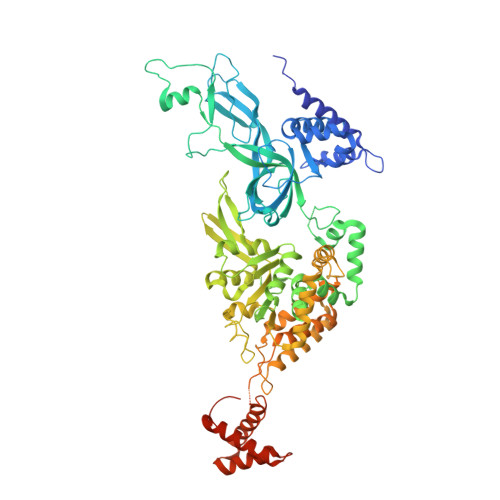
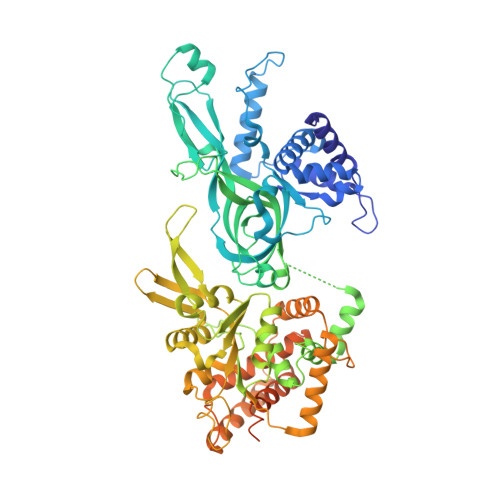
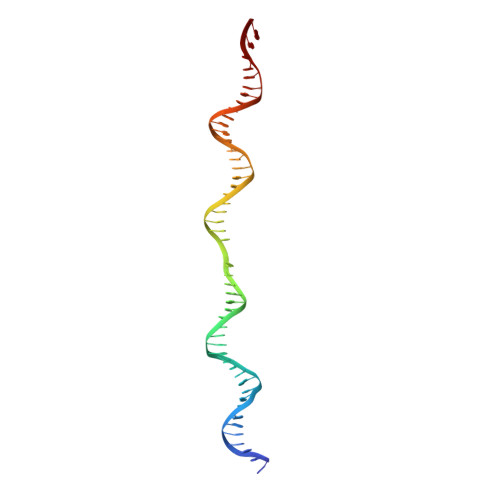
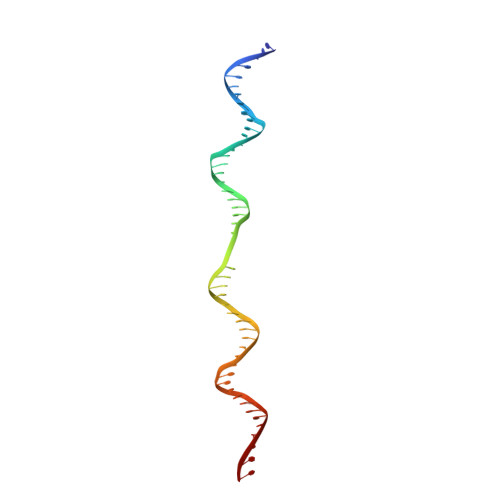
The human pre-replication complex is an open complex.
Li, J., Dong, J., Wang, W., Yu, D., Fan, X., Hui, Y.C., Lee, C.S.K., Lam, W.H., Alary, N., Yang, Y., Zhang, Y., Zhao, Q., Chen, C.L., Tye, B.K., Dang, S., Zhai, Y.(2023) Cell 186: 98-111.e21
- PubMed: 36608662
- DOI: https://doi.org/10.1016/j.cell.2022.12.008
- Primary Citation of Related Structures:
7W1Y - PubMed Abstract:
In eukaryotes, DNA replication initiation requires assembly and activation of the minichromosome maintenance (MCM) 2-7 double hexamer (DH) to melt origin DNA strands. However, the mechanism for this initial melting is unknown. Here, we report a 2.59-Å cryo-electron microscopy structure of the human MCM-DH (hMCM-DH), also known as the pre-replication complex. In this structure, the hMCM-DH with a constricted central channel untwists and stretches the DNA strands such that almost a half turn of the bound duplex DNA is distorted with 1 base pair completely separated, generating an initial open structure (IOS) at the hexamer junction. Disturbing the IOS inhibits DH formation and replication initiation. Mapping of hMCM-DH footprints indicates that IOSs are distributed across the genome in large clusters aligning well with initiation zones designed for stochastic origin firing. This work unravels an intrinsic mechanism that couples DH formation with initial DNA melting to license replication initiation in human cells.
- School of Biological Sciences, The University of Hong Kong, Hong Kong, China.
Organizational Affiliation: