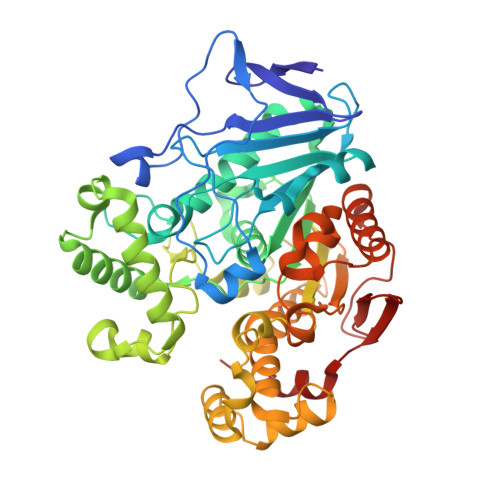
Structural Insights into (Tere)phthalate-Ester Hydrolysis by a Carboxylesterase and Its Role in Promoting PET Depolymerization
Von Haugwitz, G., Han, X., Pfaff, L., Li, Q., Wei, H., Gao, J., Methling, K., Ao, Y., Brack, Y., Mican, J., Feiler, C.G., Weiss, M.S., Bednar, D., Palm, G.J., Lalk, M., Lammers, M., Damborsky, J., Weber, G., Liu, W., Bornscheuer, U.T., Wei, R.(2022) ACS Catal 12: 15259-15270