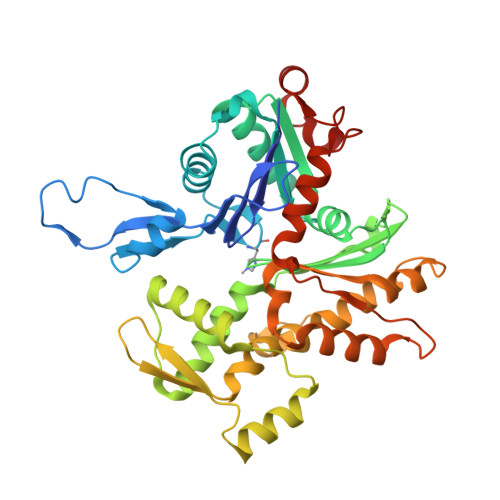
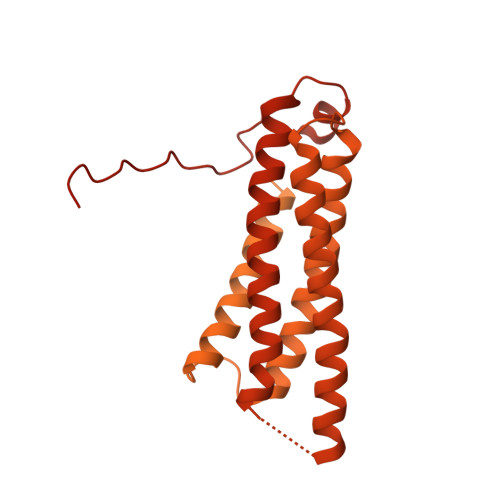
Distinct inter-domain interactions of dimeric versus monomeric alpha-catenin link cell junctions to filaments.
Rangarajan, E.S., Smith, E.W., Izard, T.(2023) Commun Biol 6: 276-276
- PubMed: 36928388
- DOI: https://doi.org/10.1038/s42003-023-04610-x
- Primary Citation of Related Structures:
7UTJ, 7UXF - PubMed Abstract:
Attachment between cells is crucial for almost all aspects of the life of cells. These inter-cell adhesions are mediated by the binding of transmembrane cadherin receptors of one cell to cadherins of a neighboring cell. Inside the cell, cadherin binds β-catenin, which interacts with α-catenin. The transitioning of cells between migration and adhesion is modulated by α-catenin, which links cell junctions and the plasma membrane to the actin cytoskeleton. At cell junctions, a single β-catenin/α-catenin heterodimer slips along filamentous actin in the direction of cytoskeletal tension which unfolds clustered heterodimers to form catch bonds with F-actin. Outside cell junctions, α-catenin dimerizes and links the plasma membrane to F-actin. Under cytoskeletal tension, α-catenin unfolds and forms an asymmetric catch bond with F-actin. To understand the mechanism of this important α-catenin function, we determined the 2.7 Å cryogenic electron microscopy (cryoEM) structures of filamentous actin alone and bound to human dimeric α-catenin. Our structures provide mechanistic insights into the role of the α-catenin interdomain interactions in directing α-catenin function and suggest a bivalent mechanism. Further, our cryoEM structure of human monomeric α-catenin provides mechanistic insights into α-catenin autoinhibition. Collectively, our structures capture the initial α-catenin interaction with F-actin before the sensing of force, which is a crucial event in cell adhesion and human disease.
- The Cell Adhesion Laboratory, UF Scripps, Jupiter, FL, 33458, USA.
Organizational Affiliation: