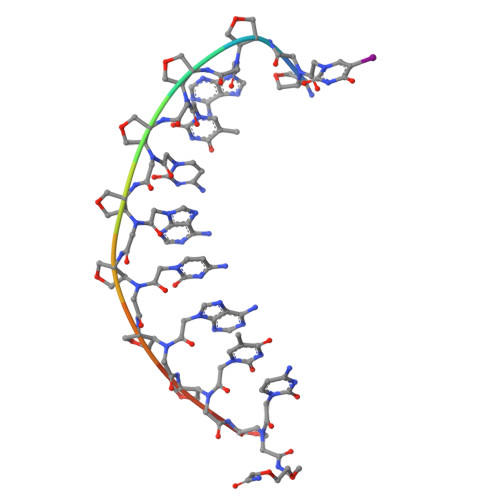
Variation of Tetrahydrofurans in Thyclotides Enhances Oligonucleotide Binding and Cellular Uptake of Peptide Nucleic Acids.
Zheng, H., Clausse, V., Amarasekara, H., Mazur, S.J., Botos, I., Appella, D.H.(2023) JACS Au 3: 1952-1964
- PubMed: 37502163
- DOI: https://doi.org/10.1021/jacsau.3c00198
- Primary Citation of Related Structures:
7UID - PubMed Abstract:
Selective incorporation of conformational constraints into thyclotides can be used to modulate their binding to complementary oligonucleotides, increase polarity, and optimize uptake into HCT116 cells without assistance from moieties known to promote cell uptake. The X-ray structure and biophysical studies of a thyclotide-DNA duplex reveal that incorporation of tetrahydrofurans into an aeg PNA backbone promotes a helical conformation that enhances binding to complementary DNA and RNA. Selective incorporation of tetrahydrofurans into the aeg PNA backbone allows polarity to be increased incrementally so that uptake into HCT116 cells can be optimized. The enhanced binding, polarity, and cellular uptake properties of thyclotides were used to demonstrate effective inhibition of microRNA-21 in HCT116 cells.
- Synthetic Bioactive Molecules Section, Laboratory of Bioorganic Chemistry (LBC), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health, 8 Center Drive, Room 404, Bethesda, Maryland 20892, United States.
Organizational Affiliation: