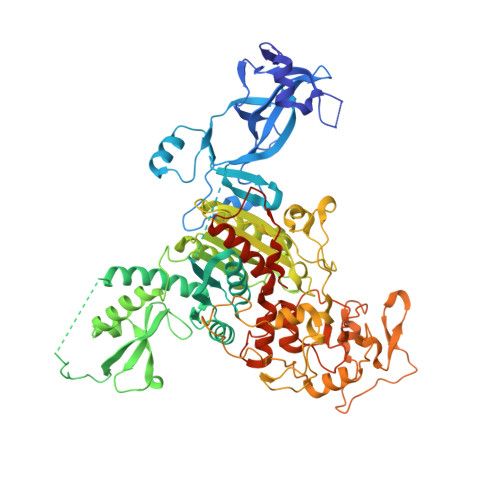
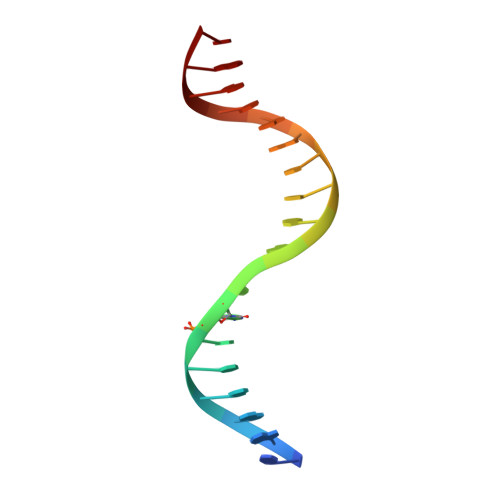
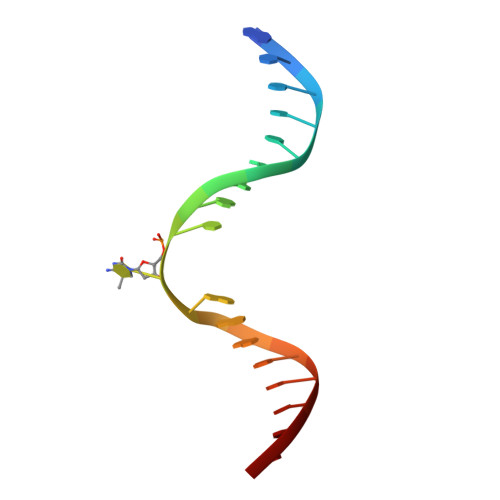
Mechanistic basis for maintenance of CHG DNA methylation in plants.
Fang, J., Jiang, J., Leichter, S.M., Liu, J., Biswal, M., Khudaverdyan, N., Zhong, X., Song, J.(2022) Nat Commun 13: 3877-3877
- PubMed: 35790763
- DOI: https://doi.org/10.1038/s41467-022-31627-3
- Primary Citation of Related Structures:
7UBU - PubMed Abstract:
DNA methylation is an evolutionarily conserved epigenetic mechanism essential for transposon silencing and heterochromatin assembly. In plants, DNA methylation widely occurs in the CG, CHG, and CHH (H = A, C, or T) contexts, with the maintenance of CHG methylation mediated by CMT3 chromomethylase. However, how CMT3 interacts with the chromatin environment for faithful maintenance of CHG methylation is unclear. Here we report structure-function characterization of the H3K9me2-directed maintenance of CHG methylation by CMT3 and its Zea mays ortholog ZMET2. Base-specific interactions and DNA deformation coordinately underpin the substrate specificity of CMT3 and ZMET2, while a bivalent readout of H3K9me2 and H3K18 allosterically stimulates substrate binding. Disruption of the interaction with DNA or H3K9me2/H3K18 led to loss of CMT3/ZMET2 activity in vitro and impairment of genome-wide CHG methylation in vivo. Together, our study uncovers how the intricate interplay of CMT3, repressive histone marks, and DNA sequence mediates heterochromatic CHG methylation.
- Department of Biochemistry, University of California, Riverside, CA, 92521, USA.
Organizational Affiliation: