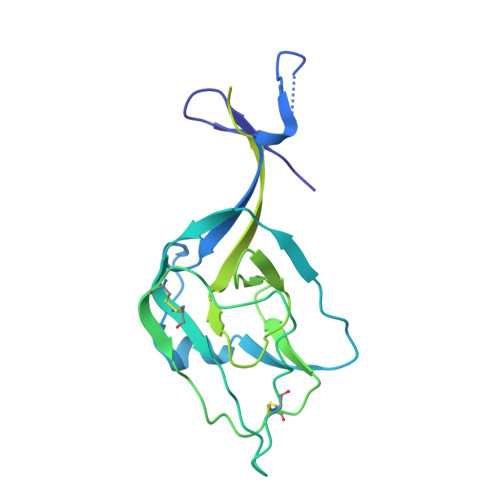
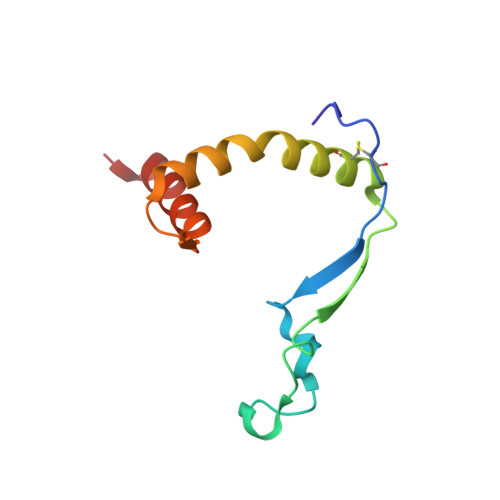
Asymmetric and non-stoichiometric glycoprotein recognition by two distinct antibodies results in broad protection against ebolaviruses.
Milligan, J.C., Davis, C.W., Yu, X., Ilinykh, P.A., Huang, K., Halfmann, P.J., Cross, R.W., Borisevich, V., Agans, K.N., Geisbert, J.B., Chennareddy, C., Goff, A.J., Piper, A.E., Hui, S., Shaffer, K.C.L., Buck, T., Heinrich, M.L., Branco, L.M., Crozier, I., Holbrook, M.R., Kuhn, J.H., Kawaoka, Y., Glass, P.J., Bukreyev, A., Geisbert, T.W., Worwa, G., Ahmed, R., Saphire, E.O.(2022) Cell 185: 995-1007.e18
- PubMed: 35303429
- DOI: https://doi.org/10.1016/j.cell.2022.02.023
- Primary Citation of Related Structures:
7N6P, 7SWD - PubMed Abstract:
Several ebolaviruses cause outbreaks of severe disease. Vaccines and monoclonal antibody cocktails are available to treat Ebola virus (EBOV) infections, but not Sudan virus (SUDV) or other ebolaviruses. Current cocktails contain antibodies that cross-react with the secreted soluble glycoprotein (sGP) that absorbs virus-neutralizing antibodies. By sorting memory B cells from EBOV infection survivors, we isolated two broadly reactive anti-GP monoclonal antibodies, 1C3 and 1C11, that potently neutralize, protect rodents from disease, and lack sGP cross-reactivity. Both antibodies recognize quaternary epitopes in trimeric ebolavirus GP. 1C11 bridges adjacent protomers via the fusion loop. 1C3 has a tripartite epitope in the center of the trimer apex. One 1C3 antigen-binding fragment anchors simultaneously to the three receptor-binding sites in the GP trimer, and separate 1C3 paratope regions interact differently with identical residues on the three protomers. A cocktail of both antibodies completely protected nonhuman primates from EBOV and SUDV infections, indicating their potential clinical value.
- Center for Infectious Disease and Vaccine Discovery, La Jolla Institute for Immunology, La Jolla, CA 92037, USA.
Organizational Affiliation: