Cryo-EM structure of human NatB in complex with CoA-Alpha-Synuclein
Deng, S., Marmorstein, R.Not Published
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Not Published
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| N-alpha-acetyltransferase 20 | 178 | Homo sapiens | Mutation(s): 0 Gene Names: NAA20, NAT5 EC: 2.3.1.254 | 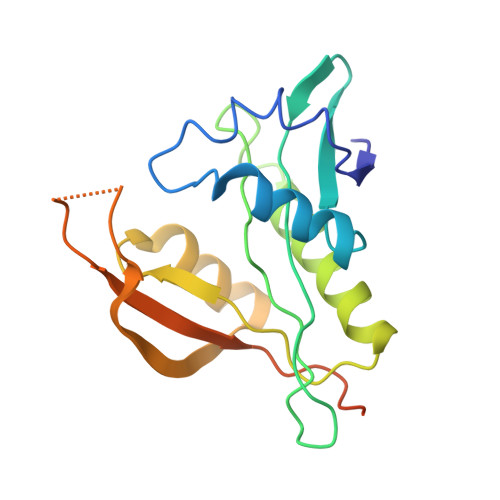 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P61599 (Homo sapiens) Explore P61599 Go to UniProtKB: P61599 | |||||
PHAROS: P61599 GTEx: ENSG00000173418 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P61599 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| N-alpha-acetyltransferase 25, NatB auxiliary subunit | 956 | Homo sapiens | Mutation(s): 0 | 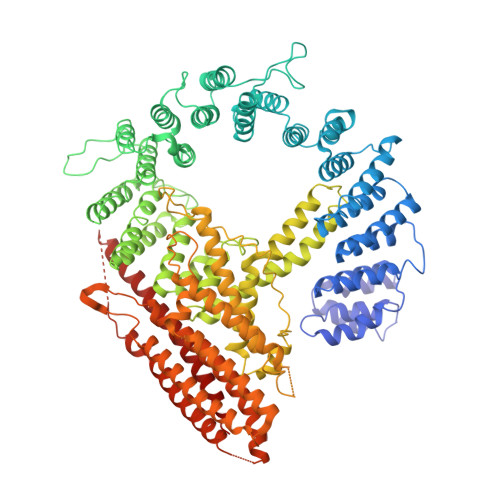 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q14CX7 (Homo sapiens) Explore Q14CX7 Go to UniProtKB: Q14CX7 | |||||
PHAROS: Q14CX7 GTEx: ENSG00000111300 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q14CX7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Alpha-synuclein | 6 | Homo sapiens | Mutation(s): 1 Gene Names: SNCA, NACP, PARK1 | 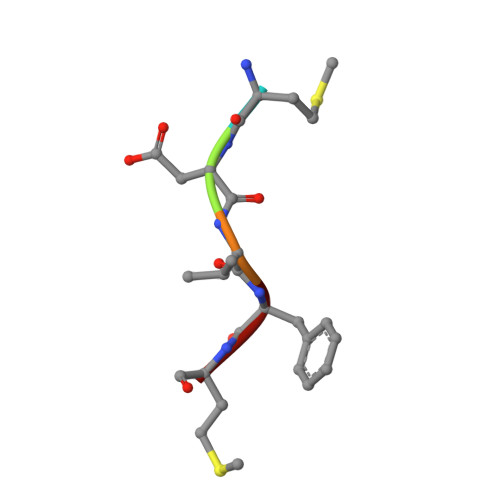 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P37840 (Homo sapiens) Explore P37840 Go to UniProtKB: P37840 | |||||
PHAROS: P37840 GTEx: ENSG00000145335 | |||||
Entity Groups | |||||
| UniProt Group | P37840 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| COA (Subject of Investigation/LOI) Query on COA | D [auth A] | COENZYME A C21 H36 N7 O16 P3 S RGJOEKWQDUBAIZ-IBOSZNHHSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.17.1-3660 |
| RECONSTRUCTION | RELION | 3.0 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | R35 GM118090 |