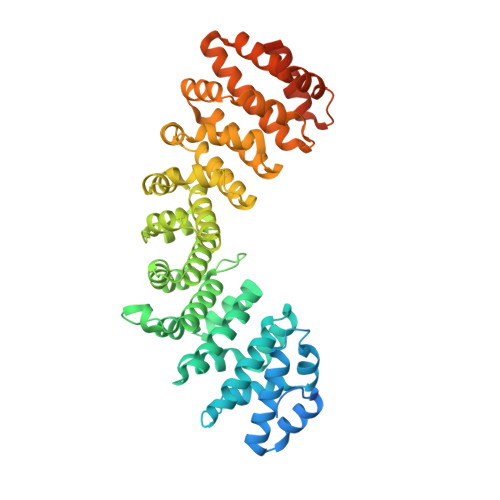
MERS-CoV ORF4b employs an unusual binding mechanism to target IMP alpha and block innate immunity.
Munasinghe, T.S., Edwards, M.R., Tsimbalyuk, S., Vogel, O.A., Smith, K.M., Stewart, M., Foster, J.K., Bosence, L.A., Aragao, D., Roby, J.A., Basler, C.F., Forwood, J.K.(2022) Nat Commun 13: 1604-1604
- PubMed: 35338144
- DOI: https://doi.org/10.1038/s41467-022-28851-2
- Primary Citation of Related Structures:
7RFX, 7RFY, 7RFZ, 7RG0, 7RG2, 7RG3, 7RG4, 7RG5, 7RG6 - PubMed Abstract:
The MERS coronavirus (MERS-CoV) is a highly pathogenic, emerging virus that produces accessory proteins to antagonize the host innate immune response. The MERS-CoV ORF4b protein has been shown to bind preferentially to the nuclear import adapter IMPα3 in infected cells, thereby inhibiting NF-κB-dependent innate immune responses. Here, we report high-resolution structures of ORF4b bound to two distinct IMPα family members. Each exhibit highly similar binding mechanisms that, in both cases, lack a prototypical Lys bound at their P2 site. Mutations within the NLS region dramatically alter the mechanism of binding, which reverts to the canonical P2 Lys binding mechanism. Mutational studies confirm that the novel binding mechanism is important for its nuclear import, IMPα interaction, and inhibition of innate immune signaling pathways. In parallel, we determined structures of the nuclear binding domain of NF-κB component p50 bound to both IMPα2 and α3, demonstrating that p50 overlaps with the ORF4b binding sites, suggesting a basis for inhibition. Our results provide a detailed structural basis that explains how a virus can target the IMPα nuclear import adapter to impair immunity, and illustrate how small mutations in ORF4b, like those found in closely related coronaviruses such as HKU5, change the IMPα binding mechanism.
- School of Biomedical Sciences, Charles Sturt University, Wagga Wagga, NSW, 2678, Australia.
Organizational Affiliation: