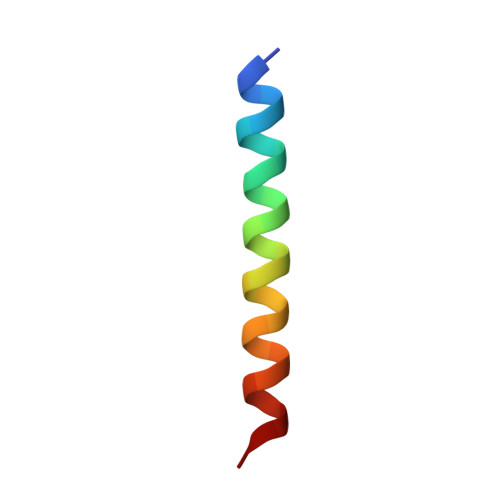
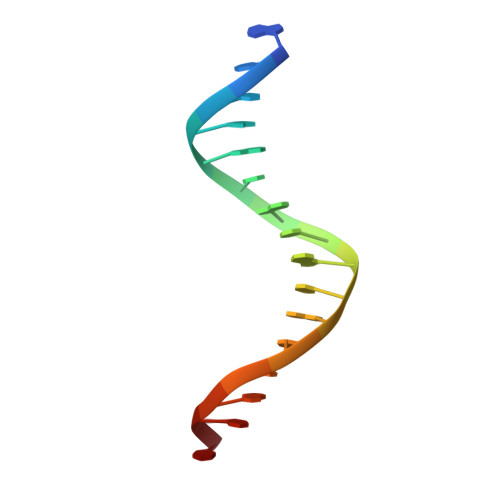
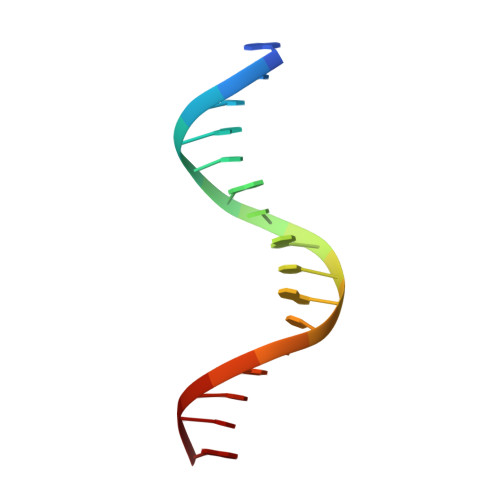
Targeting MYC with modular synthetic transcriptional repressors derived from bHLH DNA-binding domains.
Speltz, T.E., Qiao, Z., Swenson, C.S., Shangguan, X., Coukos, J.S., Lee, C.W., Thomas, D.M., Santana, J., Fanning, S.W., Greene, G.L., Moellering, R.E.(2023) Nat Biotechnol 41: 541-551
- PubMed: 36302987
- DOI: https://doi.org/10.1038/s41587-022-01504-x
- Primary Citation of Related Structures:
7RCU - PubMed Abstract:
Despite unequivocal roles in disease, transcription factors (TFs) remain largely untapped as pharmacologic targets due to the challenges in targeting protein-protein and protein-DNA interactions. Here we report a chemical strategy to generate modular synthetic transcriptional repressors (STRs) derived from the bHLH domain of MAX. Our synthetic approach yields chemically stabilized tertiary domain mimetics that cooperatively bind the MYC/MAX consensus E-box motif with nanomolar affinity, exhibit specificity that is equivalent to or beyond that of full-length TFs and directly compete with MYC/MAX protein for DNA binding. A lead STR directly inhibits MYC binding in cells, downregulates MYC-dependent expression programs at the proteome level and inhibits MYC-dependent cell proliferation. Co-crystallization and structure determination of a STR:E-box DNA complex confirms retention of DNA recognition in a near identical manner as full-length bHLH TFs. We additionally demonstrate structure-blind design of STRs derived from alternative bHLH-TFs, confirming that STRs can be used to develop highly specific mimetics of TFs targeting other gene regulatory elements.
- Department of Chemistry, University of Chicago, Chicago, IL, USA.
Organizational Affiliation: