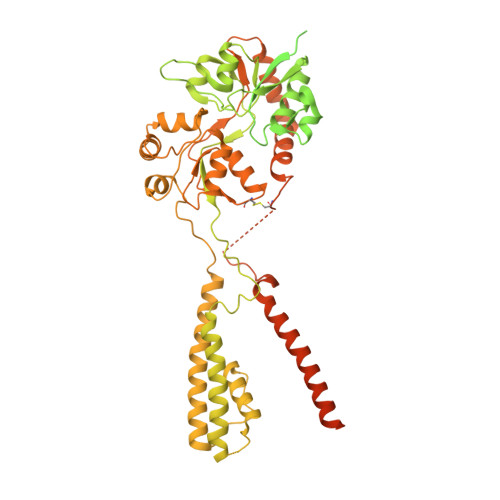
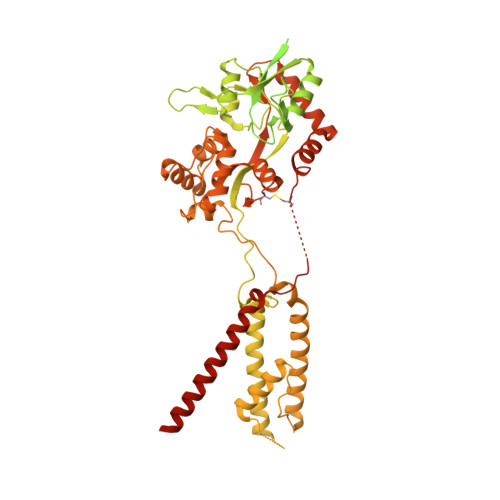
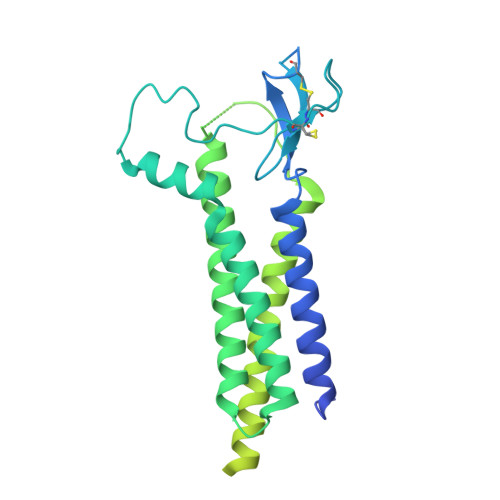
Mechanisms underlying TARP modulation of the GluA1/2-gamma 8 AMPA receptor.
Herguedas, B., Kohegyi, B.K., Dohrke, J.N., Watson, J.F., Zhang, D., Ho, H., Shaikh, S.A., Lape, R., Krieger, J.M., Greger, I.H.(2022) Nat Commun 13: 734-734
- PubMed: 35136046
- DOI: https://doi.org/10.1038/s41467-022-28404-7
- Primary Citation of Related Structures:
7QHB, 7QHH - PubMed Abstract:
AMPA-type glutamate receptors (AMPARs) mediate rapid signal transmission at excitatory synapses in the brain. Glutamate binding to the receptor's ligand-binding domains (LBDs) leads to ion channel activation and desensitization. Gating kinetics shape synaptic transmission and are strongly modulated by transmembrane AMPAR regulatory proteins (TARPs) through currently incompletely resolved mechanisms. Here, electron cryo-microscopy structures of the GluA1/2 TARP-γ8 complex, in both open and desensitized states (at 3.5 Å), reveal state-selective engagement of the LBDs by the large TARP-γ8 loop ('β1'), elucidating how this TARP stabilizes specific gating states. We further show how TARPs alter channel rectification, by interacting with the pore helix of the selectivity filter. Lastly, we reveal that the Q/R-editing site couples the channel constriction at the filter entrance to the gate, and forms the major cation binding site in the conduction path. Our results provide a mechanistic framework of how TARPs modulate AMPAR gating and conductance.
- Neurobiology Division MRC Laboratory of Molecular Biology, Cambridge, UK.
Organizational Affiliation: