Hybrid form of uridine phosphorylase from E. coli and Salmonella typhimurium in the presence PEG
Polyakov, K., Safonova, T.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Uridine phosphorylase | A [auth AAA], B [auth BBB] | 250 | Escherichia coli MS 85-1 | Mutation(s): 0 Gene Names: udp, HMPREF9350_04039 EC: 2.4.2.3 | 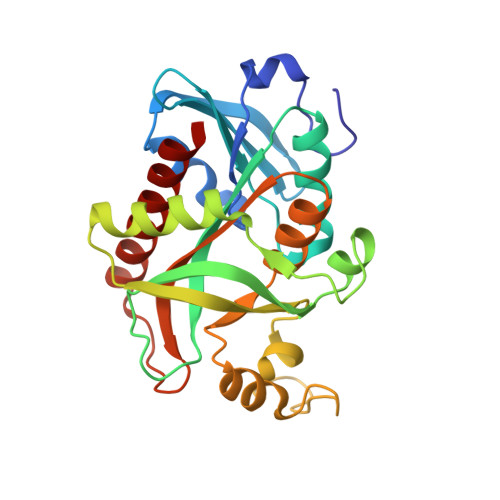 |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| FLC (Subject of Investigation/LOI) Query on FLC | D [auth AAA], E [auth BBB] | CITRATE ANION C6 H5 O7 KRKNYBCHXYNGOX-UHFFFAOYSA-K |  | ||
| K (Subject of Investigation/LOI) Query on K | C [auth AAA] | POTASSIUM ION K NPYPAHLBTDXSSS-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 150.59 | α = 90 |
| b = 150.59 | β = 90 |
| c = 46.29 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| XSCALE | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | Russian Federation | -- |