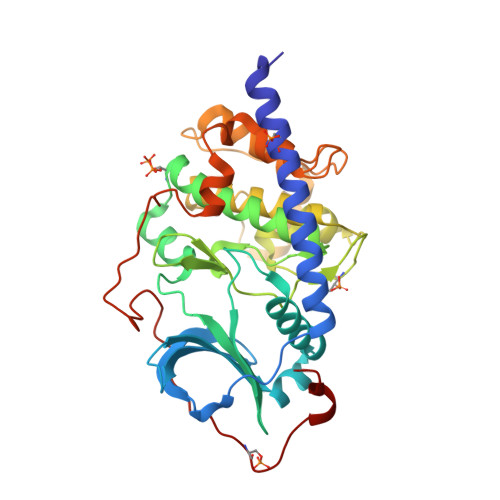
Magnet for the Needle in Haystack: "Crystal Structure First" Fragment Hits Unlock Active Chemical Matter Using Targeted Exploration of Vast Chemical Spaces.
Muller, J., Klein, R., Tarkhanova, O., Gryniukova, A., Borysko, P., Merkl, S., Ruf, M., Neumann, A., Gastreich, M., Moroz, Y.S., Klebe, G., Glinca, S.(2022) J Med Chem 65: 15663-15678
- PubMed: 36069712
- DOI: https://doi.org/10.1021/acs.jmedchem.2c00813
- Primary Citation of Related Structures:
7PID, 7PIE, 7PIF, 7PIG, 7PIH, 7PNS - PubMed Abstract:
Fragment-based drug discovery (FBDD) has successfully led to approved therapeutics for challenging and "undruggable" targets. In the context of FBDD, we introduce a novel, multidisciplinary method to identify active molecules from purchasable chemical space. Starting from four small-molecule fragment complexes of protein kinase A (PKA), a template-based docking screen using Enamine's multibillion REAL Space was performed. A total of 93 molecules out of 106 selected compounds were successfully synthesized. Forty compounds were active in at least one validation assay with the most active follow-up having a 13,500-fold gain in affinity. Crystal structures for six of the most promising binders were rapidly obtained, verifying the binding mode. The overall success rate for this novel fragment-to-hit approach was 40%, accomplished in only 9 weeks. The results challenge the established fragment prescreening paradigm since the standard industrial filters for fragment hit identification in a thermal shift assay would have missed the initial fragments.
- CrystalsFirst GmbH, Marbacher Weg 6, 35037Marburg, Germany.
Organizational Affiliation: