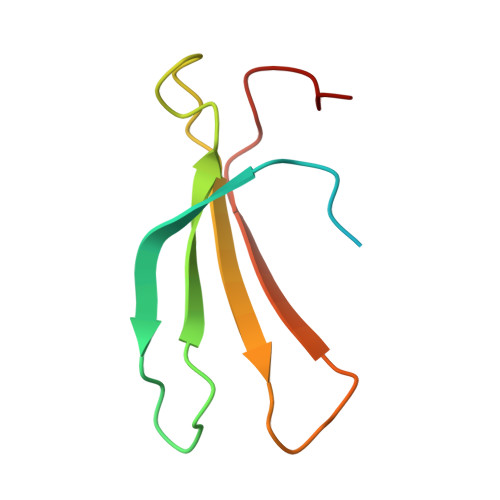
Perception of structurally distinct effectors by the integrated WRKY domain of a plant immune receptor.
Mukhi, N., Brown, H., Gorenkin, D., Ding, P., Bentham, A.R., Stevenson, C.E.M., Jones, J.D.G., Banfield, M.J.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 34880132
- DOI: https://doi.org/10.1073/pnas.2113996118
- Primary Citation of Related Structures:
7P8K - PubMed Abstract:
Plants use intracellular nucleotide-binding domain (NBD) and leucine-rich repeat (LRR)-containing immune receptors (NLRs) to detect pathogen-derived effector proteins. The Arabidopsis NLR pair RRS1-R/RPS4 confers disease resistance to different bacterial pathogens by perceiving the structurally distinct effectors AvrRps4 from Pseudomonas syringae pv. pisi and PopP2 from Ralstonia solanacearum via an integrated WRKY domain in RRS1-R. How the WRKY domain of RRS1 (RRS1 WRKY ) perceives distinct classes of effector to initiate an immune response is unknown. Here, we report the crystal structure of the in planta processed C-terminal domain of AvrRps4 (AvrRps4 C ) in complex with RRS1 WRKY Perception of AvrRps4 C by RRS1 WRKY is mediated by the β2-β3 segment of RRS1 WRKY that binds an electronegative patch on the surface of AvrRps4 C Structure-based mutations that disrupt AvrRps4 C -RRS1 WRKY interactions in vitro compromise RRS1/RPS4-dependent immune responses. We also show that AvrRps4 C can associate with the WRKY domain of the related but distinct RRS1B/RPS4B NLR pair, and the DNA-binding domain of At WRKY41, with similar binding affinities and how effector binding interferes with WRKY-W-box DNA interactions. This work demonstrates how integrated domains in plant NLRs can directly bind structurally distinct effectors to initiate immunity.
- Department of Biochemistry and Metabolism, John Innes Centre, Norwich NR4 7UH, United Kingdom.
Organizational Affiliation: