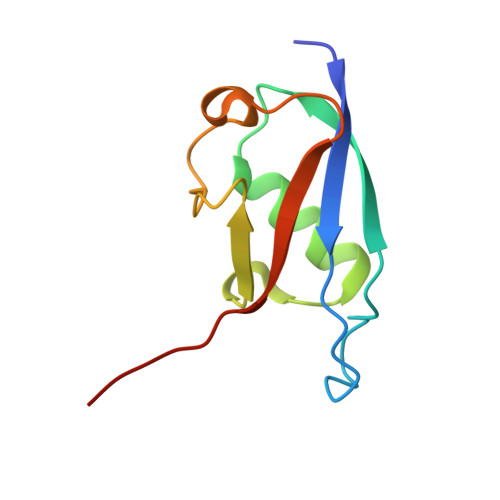
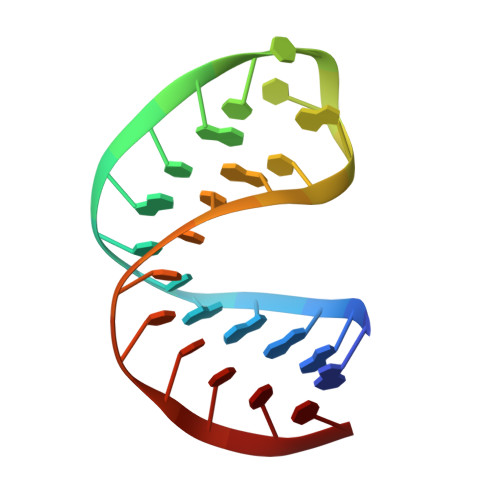
Sequence-specific RNA recognition by an RGG motif connects U1 and U2 snRNP for spliceosome assembly.
de Vries, T., Martelly, W., Campagne, S., Sabath, K., Sarnowski, C.P., Wong, J., Leitner, A., Jonas, S., Sharma, S., Allain, F.H.(2022) Proc Natl Acad Sci U S A 119
- PubMed: 35101980
- DOI: https://doi.org/10.1073/pnas.2114092119
- Primary Citation of Related Structures:
7P08, 7P0V - PubMed Abstract:
In mammals, the structural basis for the interaction between U1 and U2 small nuclear ribonucleoproteins (snRNPs) during the early steps of splicing is still elusive. The binding of the ubiquitin-like (UBL) domain of SF3A1 to the stem-loop 4 of U1 snRNP (U1-SL4) contributes to this interaction. Here, we determined the 3D structure of the complex between the UBL of SF3A1 and U1-SL4 RNA. Our crystallography, NMR spectroscopy, and cross-linking mass spectrometry data show that SF3A1-UBL recognizes, sequence specifically, the GCG/CGC RNA stem and the apical UUCG tetraloop of U1-SL4. In vitro and in vivo mutational analyses support the observed intermolecular contacts and demonstrate that the carboxyl-terminal arginine-glycine-glycine-arginine (RGGR) motif of SF3A1-UBL binds sequence specifically by inserting into the RNA major groove. Thus, the characterization of the SF3A1-UBL/U1-SL4 complex expands the repertoire of RNA binding domains and reveals the capacity of RGG/RG motifs to bind RNA in a sequence-specific manner.
- Institute of Biochemistry, Department of Biology, ETH Zürich CH-8093 Zürich, Switzerland.
Organizational Affiliation: