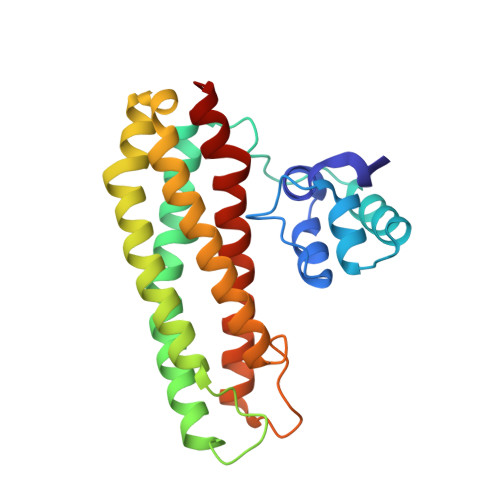
Repair of Iron Center Proteins-A Different Class of Hemerythrin-like Proteins.
Silva, L.S.O., Matias, P.M., Romao, C.V., Saraiva, L.M.(2022) Molecules 27
- PubMed: 35807291
- DOI: https://doi.org/10.3390/molecules27134051
- Primary Citation of Related Structures:
7BE8, 7OYI - PubMed Abstract:
Repair of Iron Center proteins (RIC) form a family of di-iron proteins that are widely spread in the microbial world. RICs contain a binuclear nonheme iron site in a four-helix bundle fold, two basic features of hemerythrin-like proteins. In this work, we review the data on microbial RICs including how their genes are regulated and contribute to the survival of pathogenic bacteria. We gathered the currently available biochemical, spectroscopic and structural data on RICs with a particular focus on Escherichia coli RIC (also known as YtfE), which remains the best-studied protein with extensive biochemical characterization. Additionally, we present novel structural data for Escherichia coli YtfE harboring a di-manganese site and the protein's affinity for this metal. The networking of protein interactions involving YtfE is also described and integrated into the proposed physiological role as an iron donor for reassembling of stress-damaged iron-sulfur centers.
- Instituto Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa, 2780-157 Oeiras, Portugal.
Organizational Affiliation: