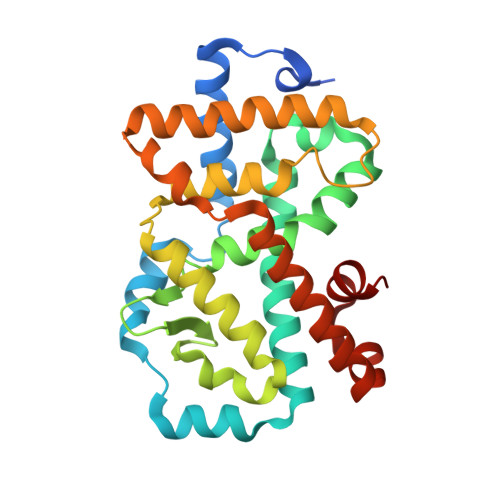
AZD0284, a Potent, Selective, and Orally Bioavailable Inverse Agonist of Retinoic Acid Receptor-Related Orphan Receptor C2.
Narjes, F., Llinas, A., von Berg, S., Jirholt, J., Lever, S., Pehrson, R., Collins, M., Malmberg, A., Svanberg, P., Xue, Y., Olsson, R.I., Malmberg, J., Hughes, G., Hossain, N., Grindebacke, H., Leffler, A., Krutrok, N., Back, E., Ramnegard, M., Lepisto, M., Thunberg, L., Aagaard, A., McPheat, J., Hansson, E.L., Chen, R., Xiong, Y., Hansson, T.G.(2021) J Med Chem 64: 13807-13829
- PubMed: 34464130
- DOI: https://doi.org/10.1021/acs.jmedchem.1c01197
- Primary Citation of Related Structures:
7OFI, 7OFK - PubMed Abstract:
Inverse agonists of the nuclear receptor RORC2 have been widely pursued as a potential treatment for a variety of autoimmune diseases. We have discovered a novel series of isoindoline-based inverse agonists of the nuclear receptor RORC2, derived from our recently disclosed RORC2 inverse agonist 2 . Extensive structure-activity relationship (SAR) studies resulted in AZD0284 ( 20 ), which combined potent inhibition of IL-17A secretion from primary human T H 17 cells with excellent metabolic stability and good PK in preclinical species. In two preclinical in vivo studies, compound 20 reduced thymocyte numbers in mice and showed dose-dependent reduction of IL-17A containing γδ-T cells and of IL-17A and IL-22 RNA in the imiquimod induced inflammation model. Based on these data and a favorable safety profile, 20 was progressed to phase 1 clinical studies.
- Mechanistic & Structural Biology, Discovery Science, R&D, AstraZeneca, Gothenburg SE-431 83, Sweden.
Organizational Affiliation: