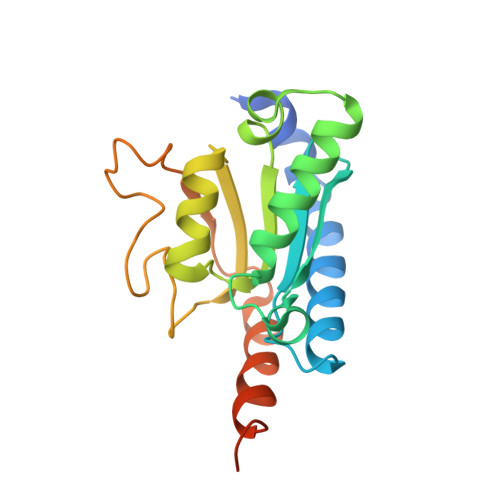
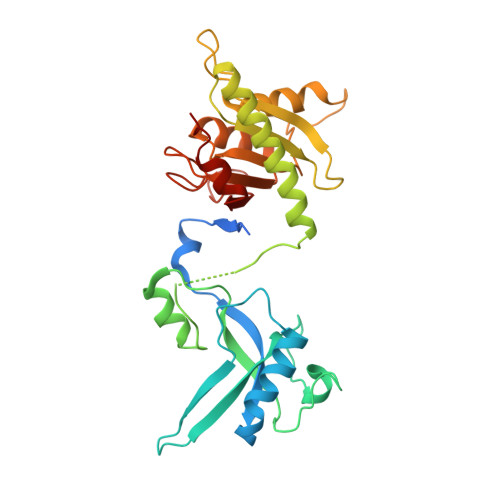
The structure of the mouse ADAT2/ADAT3 complex reveals the molecular basis for mammalian tRNA wobble adenosine-to-inosine deamination.
Ramos-Morales, E., Bayam, E., Del-Pozo-Rodriguez, J., Salinas-Giege, T., Marek, M., Tilly, P., Wolff, P., Troesch, E., Ennifar, E., Drouard, L., Godin, J.D., Romier, C.(2021) Nucleic Acids Res 49: 6529-6548
- PubMed: 34057470
- DOI: https://doi.org/10.1093/nar/gkab436
- Primary Citation of Related Structures:
7NZ7, 7NZ8, 7NZ9 - PubMed Abstract:
Post-transcriptional modification of tRNA wobble adenosine into inosine is crucial for decoding multiple mRNA codons by a single tRNA. The eukaryotic wobble adenosine-to-inosine modification is catalysed by the ADAT (ADAT2/ADAT3) complex that modifies up to eight tRNAs, requiring a full tRNA for activity. Yet, ADAT catalytic mechanism and its implication in neurodevelopmental disorders remain poorly understood. Here, we have characterized mouse ADAT and provide the molecular basis for tRNAs deamination by ADAT2 as well as ADAT3 inactivation by loss of catalytic and tRNA-binding determinants. We show that tRNA binding and deamination can vary depending on the cognate tRNA but absolutely rely on the eukaryote-specific ADAT3 N-terminal domain. This domain can rotate with respect to the ADAT catalytic domain to present and position the tRNA anticodon-stem-loop correctly in ADAT2 active site. A founder mutation in the ADAT3 N-terminal domain, which causes intellectual disability, does not affect tRNA binding despite the structural changes it induces but most likely hinders optimal presentation of the tRNA anticodon-stem-loop to ADAT2.
- Université de Strasbourg, CNRS, INSERM, Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC), UMR 7104, U 1258, 1 rue Laurent Fries, B.P. 10142, 67404, Illkirch Cedex, France.
Organizational Affiliation: