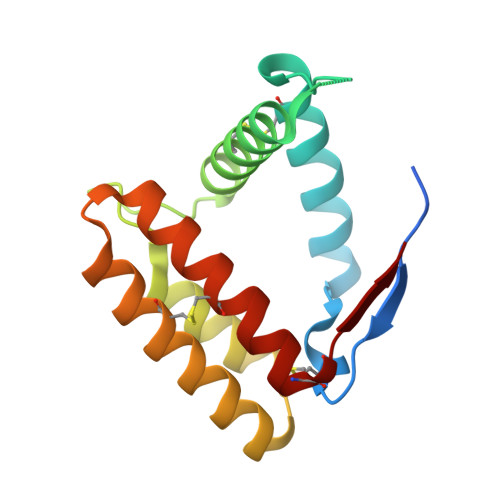
A new non-classical fold of varroa odorant-binding proteins reveals a wide open internal cavity.
Amigues, B., Zhu, J., Gaubert, A., Arena, S., Renzone, G., Leone, P., Fischer, I.M., Paulsen, H., Knoll, W., Scaloni, A., Roussel, A., Cambillau, C., Pelosi, P.(2021) Sci Rep 11: 13172-13172
- PubMed: 34162975
- DOI: https://doi.org/10.1038/s41598-021-92604-2
- Primary Citation of Related Structures:
7NYJ, 7NZA - PubMed Abstract:
Odorant-binding proteins (OBPs), as they occur in insects, form a distinct class of proteins that apparently has no closely related representatives in other animals. However, ticks, mites, spiders and millipedes contain genes encoding proteins with sequence similarity to insect OBPs. In this work, we have explored the structure and function of such non-insect OBPs in the mite Varroa destructor, a major pest of honey bee. Varroa OBPs present six cysteines paired into three disulphide bridges, but with positions in the sequence and connections different from those of their insect counterparts. VdesOBP1 structure was determined in two closely related crystal forms and appears to be a monomer. Its structure assembles five α-helices linked by three disulphide bridges, one of them exhibiting a different connection as compared to their insect counterparts. Comparison with classical OBPs reveals that the second of the six α-helices is lacking in VdesOBP1. Ligand-binding experiments revealed molecules able to bind only specific OBPs with a moderate affinity, suggesting that either optimal ligands have still to be identified, or post-translational modifications present in the native proteins may be essential for modulating binding activity, or else these OBPs might represent a failed attempt in evolution and are not used by the mites.
- Architecture et Fonction des Macromolécules Biologiques (AFMB, UMR 6098), Centre National de la Recherche Scientifique (CNRS), Aix-Marseille Université (AMU), Campus de Luminy, Case 932, 13288, Marseille Cedex 09, France.
Organizational Affiliation: